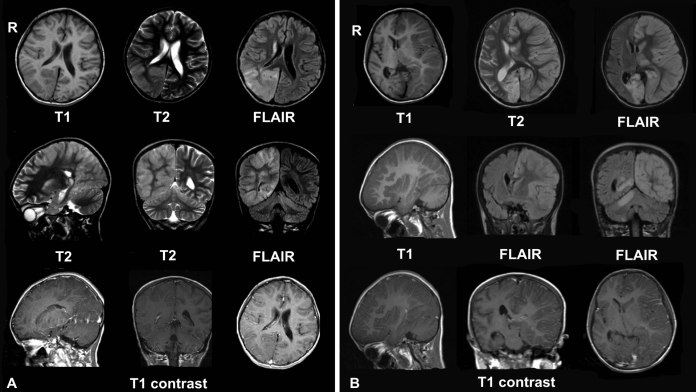
The European Medicines Agency (EMA) has commenced an urgent review of the multiple sclerosis (MS) medicine Zinbryta, following cases of serious inflammatory brain disorders.
There has been seven cases of inflammatory brain disorders, which included encephalitis and meningoencephalitis in Germany, and one case in Spain.
Following the start of the review, the company that markets Zinbryta, Biogen Idec Ltd, has informed the EMA that it intends to voluntarily withdraw the product from the market.
What is the EMA advising?
Doctors in the EU will be contacted directly in the coming days with further information, but until then the EMA advises that:
- Doctors should not start new patients on Zinbryta;
- Doctors should review patients currently treated with Zinbryta and initiate alternative therapy as soon as possible;
- Patients must not stop their medication without discussing with their doctor; and
- Patients who have any questions should talk to their doctor.
Biogen Idec Ltd has also informed the EMA of its decision to stop ongoing clinical studies with Zinbryta in the EU. Patients in the clinical studies who have any question should contact the doctor who is treating them in their study.
More about Zinbryta
Zinbryta is used for treating relapsing forms of multiple sclerosis. Following a 2017 review of the medicine’s effects on the liver, the use of the medicine was restricted to patients who have tried at least two other disease-modifying treatments and cannot be treated with any other multiple sclerosis treatments.
To date, there have been over 8,000 patients treated with Zinbryta across the globe, with most of the EU patients being treated in Germany.
Two types of inflammatory brain disorders
- Encephalitis – This is swelling of the brain caused by an infection from a virus. It can also be caused by problems with the immune system; and
- Meningoencephalitis – Bacteria that enters the bloodstream and travels to the brain and spinal cord, causing acute bacterial meningitis.









