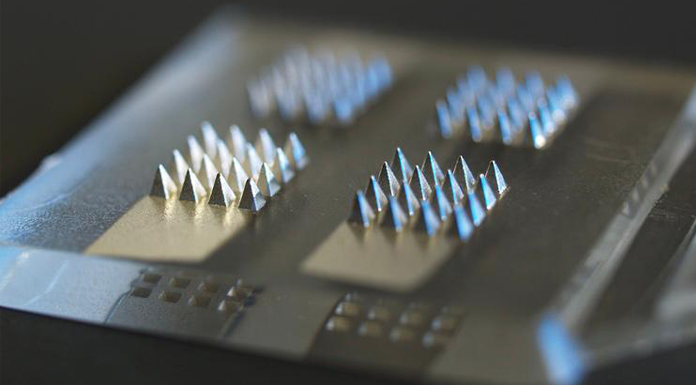
In a world first development, researchers are creating a COVID-19 smart vaccine patch that will deliver the vaccine as well as measure its efficacy.
The team at Swansea University Institute for Innovative Materials, Processing and Numerical Technologies (IMPACT) will produce the COVID-19 smart vaccine patch through the use of microneedles (MNs), which will deliver the vaccine and also measure inflammatory responses to the vaccination by monitoring biomarkers in the skin.
The project is titled Smart vaccine devices for delivery of COVID-19 vaccination and is funded by the Welsh Government Sêr Cymru funding programme. The IMPACT operation is part-funded by the European Regional Development Fund through the Welsh Government and Swansea University.
Microneedle vaccines
The patch will use tiny microneedles which are designed the break the skin barrier and deliver medicines, providing a safe and effective method to deliver a vaccine. The design allows for lower doses of the vaccine which contributes to low-cost manufacturing and simple distribution and administration. The researchers say the nature of the design would allow for a personalised approach to vaccination.
Project lead Dr Sanjiv Sharma of Swansea University said: “Measuring vaccine efficacy is extremely important as it indicates the protective effects of vaccination on an individual via the level of reduction of infection risk in a vaccinated person relative to that of a susceptible, unvaccinated individual. This measure of vaccination effectiveness will address an unmet clinical need and would provide an innovative approach to vaccine development.
“Skin vaccination using MNs has been described as a superior immunisation approach due to its potential to overcome immune tolerance observed in pregnancy, and lower vaccination costs through antigen dose-sparing, which is especially relevant in underserved countries.
“The primary goal of this project is to create a prototype of smart vaccine delivery device that can not only deliver the COVID-19 vaccine transdermally but also monitor biomarkers in the skin compartment in a minimally invasive way, offering real-time information on the efficacy of the vaccination. The new method would change the way in which vaccine efficacy trials are performed from a statistical assessment to a scientific measurement of patient inflammatory response to vaccination.
“The real-time nature of the platform will mean rapid results allowing faster containment of the COVID-19 virus. This low-cost vaccine administration device will ensure a safe return to work and management of subsequent COVID-19 outbreak waves. Beyond the pandemic, the scope of this work could be expanded to apply to other infectious diseases as the nature of the platform allows for quick adaptation to different infectious diseases.
“We are currently getting the platform ready and we hope to do human clinical studies on transdermal delivery with our existing partners at Imperial College London, in preparation for final implementation.”
Sharma added: “We are aiming to get the first prototype out by the end of the project in March 2021. We will be then looking into clinical studies and trials.”









