
Tilray’s Medical Advisory Board facilitates ground-breaking advancements in cannabinoid science.
Tilray, a Canadian federally-licensed global leader in medical cannabis research and production, is proud to announce the formation of the Tilray Medical Advisory Board, an internationally recognised group of researchers and physicians committed to facilitating ground-breaking advancements in the emerging field of cannabinoid science. Since its first meeting in June 2017, the board has been evaluating opportunities for medical cannabis research and clinical trials in conjunction with leading hospitals and universities worldwide.
“As public interest in cannabis-derived medicines continues to grow, we need objective, valid data about the potential risks and benefits of medical cannabis gathered through clinical trials and the scientific method,” said Dr Orrin Devinsky, MD, Chairman of the Tilray Medical Advisory Board and Director of the Comprehensive Epilepsy Center at NYU Langone Medical Center, US. Dr Devinsky was the lead investigator for the first study approved by the US Food and Drug Administration (FDA) to evaluate the efficacy of a cannabis-based drug for paediatric epilepsy patients.
“Clinical research is critical to furthering our understanding of how patients might use cannabinoid-based medicines to treat a range of symptoms and conditions, including pain, epilepsy and symptoms associated with cancer. Early research has also indicated potential therapeutic value in rheumatoid diseases, slowing certain cancer growth and dermatological conditions,” Dr Catherine Jacobson, PhD, Director of Clinical Research, who works closely with the board to evaluate research opportunities, added.
Additional members of the Tilray Medical Advisory Board are:
- Praveen Anand, MD – Head of the Centre for Clinical Translation and Professor of Clinical Neurology at Imperial College London, UK. His research focuses on pathophysiological and molecular mechanisms in human sensory neuropathies and chronic pain syndromes;
- Abraham Chachoua, MD – Associate Director of Cancer Services at NYU Langone Perlmutter Cancer Center and Jay and Isabel Fine Professor of Oncology at NYU Langone Department of Medicine, US. He specialises in the treatment of cancers that affect the lungs and chest;
- Elizabeth K Hale, MD – Clinical Associate Professor of Dermatology at NYU Langone Medical Center and co-founder of CompleteSkinMD. She has extensive experience in the field of skin cancer and is a Senior Vice-President of the Skin Cancer Foundation; and
- Catherine Lord, PhD – Professor of Psychology in Psychiatry and founding Director of the Center for Autism and the Developing Brain (CADB), a collaborative programme between New York Presbyterian Hospital, Weill Cornell Medicine, and Columbia University College of Physicians and Surgeons in partnership with New York Collaborates for Autism. She is internationally recognised for her work in longitudinal studies of children with autism, as well as for her role in developing the autism diagnostic instruments used in both practice and in worldwide research today.
Tilray European Union Campus
In step with the launch of the Medical Advisory Board, Tilray – Canada’s first federally-licensed cannabinoid producer to obtain EMA GMP Certification issued by an EU Competent Authority – is investing in the European Union to serve the continent’s patients efficiently.
Tilray made history in September 2017 by announcing it will invest up to €20m in a European Union Campus after receiving licences from the Government of Portugal to import cannabis genetics and to cultivate cannabis for medical purposes. This is the first time a medical cannabis producer has been licensed to cultivate on multiple continents, and over the next three years, the Tilray project is expected to create 100 direct jobs, including highly skilled positions.
In early November, Tilray became the first company to import live cannabis genetics to Europe.
“Tilray’s EU Campus is another strategic milestone as we aim to build the world’s most trusted and admired medical cannabis brand,” said Brendan Kennedy, CEO of Tilray. “For the past two years we’ve been working hard to find the right location for cultivation, processing, and research facilities to serve rapidly growing demand for high-quality medical cannabis products in Europe. Portugal has the ideal climate to cultivate cannabis, a highly skilled healthcare workforce, and a vibrant research community. It’s more environmentally friendly and cost-effective to supply European patients from Portugal than from northern climates.”
Until the Tilray announcement of its landmark full-service EU Campus, Europeans had few cannabinoid options besides a limited availability of dried cannabis as the starting material for compounding in pharmacies. The supply of plant material was outpaced by European demand and patients were often left in a lurch as appropriate dosage forms remained out of reach.
But now, Tilray’s EU Campus offers partners across the bloc a full range of products and services, from cultivation and drug product formulation to wholesale supplies of active pharmaceutical ingredients or a pan-European cannabinoid product distribution network.
Tilray will invest up to €20m in multiple facilities located in and around the BIOCANT Research Park in Cantanhede, Portugal. The Tilray EU Campus will include indoor, outdoor and greenhouse cultivation sites, as well as facilities to process, package and distribute medical cannabis and cannabinoid-derived medical products. As a part of BIOCANT, the EU Campus will serve as a hub supporting Tilray’s clinical research and product development efforts across Europe.
To be ready to serve Europe in a timeframe that matters, Tilray has secured laboratory and indoor cultivation space within BIOCANT and the company has already begun construction on a greenhouse and a processing facility on property purchased in Portugal.
Phase one of Tilray’s EU Campus development initiative, which is expected to be complete by spring 2018, includes an indoor laboratory and genetics bank, outdoor cultivation sites, a 10,000m2 greenhouse, and a 1,500m2 processing facility.
Subsequent phases, which are expected to be completed by 2020, will add 15,000m2 of greenhouse cultivation space and another 1,500m2 for processing.
A clinical overview of cannabis
Cannabinoids and the endocannabinoid system
Cannabis sativa contains hundreds of cannabinoids. The two most well-studied and prevalent cannabinoids are tetrahydrocannabinol (Δ9THC) and cannabidiol (CBD). Studies have shown that THC is responsible for the euphoric effect commonly associated with cannabis. While there is ongoing research on other minor cannabinoids present in cannabis, their physiological relevance is still largely unknown.
The endocannabinoid system (ECS) is a complex system of receptors, ligands and enzymes contained within the body. The ECS has been shown to play a role in fundamental biological processes such as pain modulation, movement control, energy balance, mood and memory.4 The phytocannabinoids THC and CBD act within this ECS, such as on the CB1R and CB2R, but have also been shown to modulate the activity of receptors outside the ECS, such as GPR55, TRPV1 and GPR119.5 As the body of research on the importance of the ECS grows, so does interest in developing novel chemical entities (NCEs) that target pathways involved in regulating fundamental processes modulated by the ECS. It is critical to note that these NCEs are distinct from the phytocannabinoids THC and CBD discussed in this article.
Cannabinoid Products
Typically, when we speak of drug therapy, we imply two factors. The first is a definition of the drug itself: a formulation with a defined active ingredient (or combination of active ingredients) shown to be responsible for a therapeutic effect. The second is clinical data to inform treatment with that drug product. Yet the term ‘medical cannabis’ has come to represent a range of products, from inhaled whole dried flower to orally administered pharmaceutical products containing defined doses of cannabinoids. This range in definition, understandably, creates significant confusion for prescribing physicians.
The most common methods of the administration of cannabinoids are via the lungs by inhaling or via the gut by swallowing oral solutions or capsules. Cannabis strains can vary dramatically in cannabinoid content and composition, resulting in a highly variable dose of the active ingredient. While there have been many published studies on inhaled cannabis flower, the results of these studies are difficult to interpret.
In the last 30 years, pharmaceutical drug products containing THC, or THC and CBD in combination, including Marinol (Dronabinol), Cesament (Nabilone), and Sativex (Nabiximols), have been approved for several indications. However, even in countries where federal health agencies have approved their use, these pharmaceutical cannabinoid medications are often rarely accessed by patients. In many EU countries, this is partly due to the onerous documentation requirements imposed on prescribing physicians and partly due to the high cost and non-reimbursable nature of the drugs.
Cannabinoid pharmacokinetics
Cannabinoid pharmacokinetics are highly influenced by the route of drug administration and the formulation of the drug product. Inhalation acts as an efficient and rapid method of delivery, whereas oral ingestion results in slower absorption with lower, more-delayed peak THC and CBD plasma levels. The variability of both THC content in plant material (0.3% to 30%) and inhalation dynamics leads to unpredictable plasma THC levels from smoking.
Reported Cmax and area under the curve (AUC) values of THC and CBD from inhalation are one to two orders of magnitude higher than via the oral route. Tmax after inhalation is substantially shorter (0.14 to 0.26 hours) compared to oral administration.
The bio-availability of THC and CBD is, on average, two to threefold higher upon inhalation than upon oral ingestion. Low oral bio-availability may be the result of variations in drug absorption and degradation resulting from different physiological states (full vs. empty stomach) and first-pass metabolism in the liver.4
One of the most critical issues facing the future of cannabinoid treatments is the development of formulations that do not require smoking or vaporisation. While cannabis has traditionally been ingested via pulmonary delivery, there are several disadvantages to this method. First, the chemical composition of whole cannabis flower varies widely and is rarely accounted for. Second, the patient is exposed to toxic by-products of combustion or potentially harmful additives found in cannabis concentrates processed for vaporisation. Third, pulmonary delivery has a specific pharmacokinetic profile that is not suited for many of the indications for which cannabinoids may be effective. If we are to develop best-in-class cannabinoid treatments, the pharmacokinetic parameters of investigational drug formulations must be considered.
Therapeutic versus medical use
Reports describing the effects of cannabis on various symptoms fall into two principal groups: those describing the effects of inhaled whole cannabis flower (or its preparations) and those using isolated cannabinoids. This distinction is critical since the former is closely linked to the historical use of cannabis as a herbal medicine. This use is still considered therapeutic in some communities and relies on the patient’s ability – via trial and error – to discover products that relieve symptoms and establish the most effective use of those products. The physician is rarely involved in guiding the patient in this type of use.
In contrast, the use of drug products containing defined doses of active ingredients is largely driven by conventional pharmaceutical development of specific cannabinoids such as THC or CBD. This truly medical use requires clinical data to assist the physician in guiding appropriate treatment: data such as a safe starting dose and titration schedule, side effects profiles, drug-drug interactions and efficacy for specific indications.
Clinical evidence
The medical cannabis literature is far from satisfactory. It contains many small-scale studies, open-label trials and case studies, but very few good quality, statistically significant placebo-controlled, blinded trials – the gold standard for proving efficacy. Clinical research on cannabinoids has been limited by the products available for research. Drawing solid conclusions from the literature can be difficult.
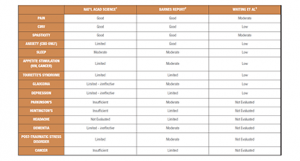
Meta-analyses and commissioned reports have attempted to bring some order to the chaos by categorising existing trials by methodological validity. For this article, results from one published meta-analysis and two major reports, commissioned by national public institutions, are included (Fig. 1). Each of these reports graded existing trials using all types of medical cannabis products according to methodological validity – rating randomised placebo controlled clinical trials (RCTs) as the most robust and open-label trials and case studies as the least robust. A range of indications were evaluated.
Whiting et al. published a meta-analysis reviewing 79 trials (screened from an initial 505 identified studies).3 GRADE (Grading of Recommendations Assessment, Development and Evaluation) was used to rate the overall quality of the evidence for risk of bias, publication bias, imprecision, inconsistency, indirectness and magnitude of effect. The indications reviewed by Whiting et al. were prespecified by project funder the Swiss Federal Office of Public Health.
A 2016 report was commissioned by the All-Party Parliamentary Group (APPG) for Drug Policy Reform in the UK, wherein the goal was to ‘to assess the evidence and bring a dispassionate view of what is known about current and potential applications of cannabis-based treatments’ (Barnes Report, 2016).2 The authors of this report summarised the clinical evidence from ~120 studies, culled from over 2,200 initially identified, adopting the grading system used by the American Academy of Neurology.7
In early 2017, a group of leading researchers in the field were tasked with conducting a comprehensive review of current evidence on the health effects of cannabis and cannabinoids by the US National Institutes of Science, Engineering and Medicine.1
In addition to summarising the evidence for the therapeutic potential of cannabis, this report also lays out the potential harmful health effects of use. The strongest clinical evidence for efficacy appears to be for the treatment of various types of pain, nausea and vomiting associated with chemotherapy, spasticity associated with multiple sclerosis (MS), and anxiety. It is important to note that, for anxiety, products containing only CBD are indicated; THC can cause an increase in anxiety in ~10% of the population. Of note, the National Academies report found that there is some evidence on a lack of efficacy for both glaucoma and depression, whereas the other two reports found evidence in support of efficacy, illustrating the difficulties in interpreting small-scale clinical studies with different formulations of the active ingredient(s).
New evidence since the publication of these reports indicates that CBD may be effective in treating some seizure types. Recent data published in the New England Journal of Medicine indicates that an investigational drug product containing the single active ingredient CBD (Epidiolex®; GW Pharma) results in a statistically significant reduction in seizure frequency.8 The data on combination drug products containing CBD and THC for epilepsy are thus far inconclusive, though there are widespread reports of artisanal cannabis preparations in use for the treatment of seizures. One of the most critical areas of investigation remains the study of combination THC/CBD drug products in the treatment of specific seizure types.
Common reported side effects associated with cannabinoid use were dizziness, dry mouth, nausea, fatigue, euphoria, vomiting, disorientation, drowsiness, confusion, loss of balance, and hallucination. Most side effects were reported to be mild at low doses, increasing in severity and frequency as doses escalated, and resolved upon discontinuation of the study drug.
Cautions and risks
The Barnes and the National Academies reports suggest that there is some evidence for a causal link between cannabis use and schizophrenia, particularly in those individuals with a genetic predisposition to psychosis or among the most frequent cannabis users. Thus, caution is recommended when prescribing cannabinoid medicines for such individuals. There is a small dependency rate with cannabis – at around 9% – which warrants caution. This compares to a 15% dependency rate for alcohol and 32% dependency rate for tobacco. The evidence for cognitive impairment for long-term use is not clear, especially in the developing brain. As with all prescription drugs, a careful risk/benefit analysis is warranted, taking into account the severity of symptoms being treated by the drug and its side effects.
The effects of THC on driving must be studied so that informed policies can be put in place to ensure public safety. Therapeutic doses of cannabinoids are significantly lower than the amounts traditionally associated with recreational use. Thus, the assessment of potential long-term harmful physiological effects and risks on public health should focus on therapeutic doses of medical cannabis preparations as opposed to commonly used recreational doses.
Conclusion
There is considerable literature demonstrating the efficacy of cannabinoid therapy for various types of pain, chemotherapy-induced nausea and vomiting, spasticity associated with MS, and anxiety.
There remains a great need for larger, adequately powered and controlled studies evaluating the efficacy of cannabinoids for other indications. As the field moves forward, particular attention must be paid to the specific composition of the active ingredient/s best suited to treat specific symptoms, as well as the pharmacokinetic profile of the drug product. Further studies on the potential risks and health hazards associated with cannabis use are critical so that vulnerable patients can be protected, particularly from longer-term negative effects. These studies will be facilitated by the legalisation of cannabinoids for medical indications in strictly controlled circumstances with the development of quality-controlled products.
References
1 The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research.
https://www.nap.edu/catalog/24625/the-health-effects-of-cannabis-andcannabinoids-the-current-state
The National Academies of Sciences, Engineering, and Medicine. Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda (2017). www.nap.edu
2 Cannabis: The Evidence for Medical Use (2016) Professor Michael P Barnes MD FRCP Honorary Professor of Neurological Rehabilitation, Newcastle University, and Dr Jennifer C, Barnes D, Psychol Clinical Psychologist, Northumberland, Tyne & Wear NHS Foundation Trust. http://www.drugpolicyreform.net/
3 Whiting PF, RF Wolff, S Deshpande, M Di Nisio, S Duffy, AV Hernandez, JC Keurentjes, S Lang, K Misso, S Ryder, S Schmidlkofer, M Westwood, J Kleijnen. (2015) Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. Journal of American Medical Association 23-30;313(24):2456-73
4 Oier, AO, E Izaskun, RB Irantzu, Z Iratxe, E Nestor, U Aresatz. (2017) Targeting the endocannabinoid system: future therapeutic strategies. Drug Discovery Today 22(1): 105-110
5 De Petrocellis, L, A Ligresti, AS Moriello, M Allara, T Bisogno, S Petrosino, CG Scott, V Di Marzo. (2011) Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. British Journal of Pharmacology 163.7: 1479–1494
6 Huestis, M (2007) Human Cannabinoid Pharmacokinetics. Chemistry & Biodiversity 4: 1770–1804
7 Koppel, BS, JC Brust, T Fife, J Bronstein, S Youssof, G Gronseth, D Gloss. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 29; 82(17): 1556-63 (2014)
8 Devinsky, O, JH Cross, L Laux, E Marsh, I Miller, R Nabbout, IE Scheffer, EA Thiele, S Wright (2017) Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. New England Journal of Medicine 376(21):2011-2020











