
A collaborative study, including the University of Chicago Medicine, has discovered a link between a set of sporadic genetic mutations and lesions associated with strokes.
A rare type of brain blood vessel malformation known as a cavernous angioma impacts more than one million Americans and carries a lifetime risk of stroke and seizures. Only around one-third of cases can be connected to inherited familial sporadic genetic mutations, and until now, their cause was unknown.
How was this link identified?
The University of Chicago Medicine, Duke University, and the University of Pennsylvania has collaboratively worked together in order to investigate sporadic genetic mutations, along with additional mutations in the same area that fuel the lesion’s growth.
Understanding the underlying causes of these brain malformations will be the key to discovering which patients are at risk for their development and finding effective treatments for the condition.
This study was published on 14 March 2022 in Nature Cardiovascular Research.
“We have known for more than two decades that there is a familial form of cavernous angiomas that is inherited via genes passed on from generation to generation,” said Issam Awad, MD, the John Harper Seeley Professor of Neurological Surgery, and Director of Neurovascular Surgery at University of Chicago Medicine. “But in the majority of people with this type of brain bleeding, the lesion is not inherited. And until now, we have never known why some people randomly end up with this lesion.”
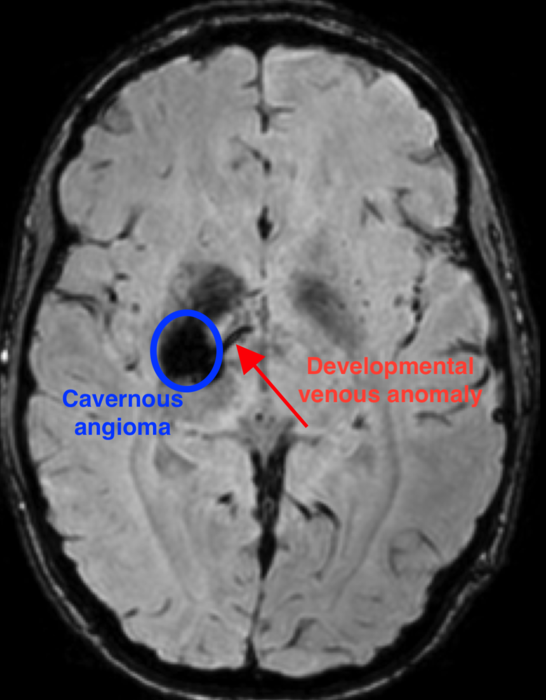
Photo Credit: Issam Awag, MD.
What have scientists determined regarding sporadic genetic mutations?
Researchers were able to identify a unique combination of sporadic genetic mutations that occurs during the development of the brain that results in a cavernous angioma. First, a mutation in the gene – known as PIK3CA – leads to an abnormal pattern of vessels in the brain, known as a developmental venous anomaly (DVA).
The DVA alone is generally innocuous. But when a second mutation in one of several genes, such as MAP3K3, KRIT1, CCM2, or PDCD10, occurs in the area of the abnormal vein, a cavernous angioma develops.
“We had previously observed that often these lesions grow near a pre-existing abnormal vein,” explained Awad. “But these DVAs are actually very common – about 6% to 10% of people have one, and the vast majority of them never have any problems.
“Rarely, those veins grow a cavernous angioma and we have never known why. In this study, we were finally able to use mutation analysis on the vein itself, to see why the vein seems predisposed to these angiomas.”
The researchers were able to examine the genetics of both the angioma as well as its connected DVA, due to the delicate surgical method utilised to repair bleeding lesions. This requires removing small portions of the veins to detangle them from the cavernous angioma lesion. This led to the discovery of the genetic mutation in PIK3CA in the vein, and the realisation that the same mutation co-occurs with a second mutation within the angioma.
“This is very novel, because we can now explain why the DVA forms in the first place,” noted Awad. “Along with a second mutation, it is the genetic seed for the formation and growth of the cavernous angioma.”
Additionally, as well as the discovery of a genetic mechanism for the formation of the DVA, the research team also discovered molecules circulating in the blood that are associated with the key brain mutation. This is the first time that a blood test for a focal somatic mutation in the brain has been revealed.
“Now, we can develop blood tests that can identify these mutations in the brain, and in the future, we can develop therapies that can inhibit the mechanisms that cause these lesions to form,” Awad added. “Some of the genes we have identified can be inhibited by drugs that are already on the market.”
How will this be integrated into healthcare?
The researchers intend to translate these findings into additional research and, ultimately, more treatments to prevent and heal cavernous angiomas. To achieve this, scientists will search for biomarkers that might help distinguish benign DVAs from the ones that are destined to grow a cavernous angioma.
“Ideally, we will be able to tell with a simple blood test if you have a benign vein abnormality, or if it has the seed that will lead it to grow an angioma,” concluded Awad. “In addition, we will be testing some of these pharmacologic inhibitors of the mutations we have identified to see if they will stabilise or even shrink the brain lesions.
“A mechanism is not just about scientific curiosity. It should motivate us to change patient care. If we do not know the mechanism, we cannot have a truly rational therapy.”









