For the first time, researchers have shown that the protein interface that drives cancer growth could act as a target for more effective treatments.
Led by the Science and Technology Facilities Council’s Central Laser Facility, the study used advanced laser imaging techniques to identify structural details of a mutated protein that drives cancer growth by evading drugs that target it.
The study lays the groundwork for future research into more effective and long-lasting cancer therapies.
About the protein
The Epidermal Growth Factor Receptor is a protein that sits on the surface of cells and receives modular signals that tell the cell to grow and divide.
Mutated EGFR stimulate uncontrolled growth in certain types of cancer, resulting in tumours.
Current cancer treatments are limited
Many cancer treatments block and inhibit mutant EGFR to prevent the formation of tumours.
However, these are limited as cancerous cells develop further EGFR mutations that are resistant to treatment.
Until now, how these drug resistant EGFR mutations drive cancer growth was not understood, hindering the ability to develop treatments that target them.
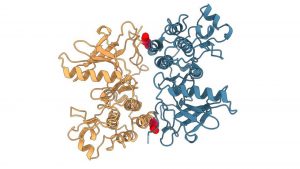
FLImP analysis
In the new study, CLF scientists obtained super-resolution images of a drug-resistant EGFR known to contribute to lung cancer. They achieved this by using an advanced laser imaging technique called Fluorophore Localisation Imaging with Photobleaching (FLImP).
The FLImP analysis revealed structure details as small as two nanometres. The image showed how molecules in the drug-resistant EGFR mutation interact.
A team from the University of Geneva used advanced computer simulations, combined with the FLImP analysis, to provide atomistic details of the mutant EGFR complexes.
These were then used to compare the structural details of the mutated and healthy EGFR to identify interfaces between interacting molecules in the drug-resistant mutation critical for tumour growth.
Professor Marisa Martin-Fernandez, Leader of the Octopus Group at CLF, which led the study, said: “If this interface proves to be an effective therapeutic target, it could provide an entirely new approach to much-needed pharmaceutical development.”
Introducing additional mutations to block cancer growth
The team then introduced additional mutations to the drug-resistant EGFR in cultured lung cells and in mice that interfered with the newly discovered interfaces.
In these experiments, one of the additional EGFR mutations was shown to block cancer growth, with the mice developing no tumours. This further indicated that this EGFR mutation’s ability to promote cancer depends on these interfaces.
Potential for new cancer therapies
The team hope that the interfaces could act as potential targets for new cancer therapies to prevent further growth.
CLF is continuing to test the research method on other EGFR mutations known to contribute to lung cancer.
They also hope to establish whether the protein interface plays a role in the development of other cancers.









