The dramatic increase in age-associated disorders such as cardiovascular disease represents a major burden on modern society. Researchers at the University of Leuven, Belgium, are working to prevent this by improving our understanding of the mechanisms behind cardiac dysfunction.
In Europe, cardiovascular disease is the leading cause of death with over 3.9 million deaths a year, or 45% of all deaths, and more than 85 million people are living with symptomatic cardiovascular disease such as ischaemic heart disease or heart failure. For instance, late-stage heart failure (when disease presents with symptoms and patients are often hospitalised) affects over 15 million Europeans.1
Any form of symptomatic heart failure has poorer prognosis than many forms of cancer. The cost of medical care of symptomatic heart failure is measured in the billions of euros. Damage to the heart can also affect multiple organs and occurs with other severe health problems and, therefore, significantly affects wellbeing. Professor Eugene Braunwald, one of the most prominent cardiologists of our time, compared the treatment of acute cardiovascular events to the process of “locking the barn door after the horse is stolen”. Therefore, one of the most effective approaches for large-scale prevention of cardiovascular disease is to better understand the complexity of determinants leading to cardiac dysfunction and an improvement in early detection of this condition.
In this context it should be stressed that heart failure is a progressive condition that:
- Begins with risk factors for heart dysfunction (e.g. hypertension, obesity and diabetes);
- Proceeds to asymptomatic maladaptive heart remodelling and dysfunction; and
- Evolves into clinically overt heart failure, disability and death.
Timely recognition of earlier stages of cardiac maladaptation will have a crucial impact on preventive treatment strategies and an individual’s length and quality of life. However, an integral platform including clinical, behavioural, imaging and -omics data for early detection of early stages of heart failure and for prediction of its complications is lacking.
Ongoing at KU Leuven
Our epidemiological work at the KU Leuven Department of Cardiovascular Sciences, Belgium, focuses on improving our understanding of trajectories from normal cardiac structure and function to heart failure and our predictions of the onset and severity of this disease. This project utilises well-phenotyped general population cohorts with a series of cardiovascular imaging and outcome data: the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO) and European Project on Genes in Hypertension (EPOGH). These cohorts are among the largest and best characterised studies on cardiovascular risk factors and outcomes, as well as progression of left ventricular remodelling/dysfunction and co-morbidities in the general population, with modern phenotyping especially of macro- and microvascular dysfunction and subclinical (early) organ damage. We also gather a broader picture of variation in biomarker profiles in relation to cardiac phenotypes. We aim to expand our analysis to larger population cohorts and additional clinical settings, which will enrich not only the predictive value of the data we generate but also its potential to yield mechanistic insights into disease.
Cardiac imaging – the potential of data
The current diagnostic imaging algorithm used in the clinic to detect cardiac dysfunction focuses on the late (symptomatic) stage of heart failure and, therefore, is not effective to identify patients at risk of cardiovascular outcomes. For instance, the application of the recent recommendations led to a lower prevalence of subclinical left ventricular diastolic dysfunction in the high-risk population and to an underestimation of cardiovascular risk in asymptomatic subjects. Therefore, one aspect of our work focuses on redefining and validating the best imaging criteria for detection of the early stages of left ventricular malfunction in the community. From preliminary analysis of the FLEMENGHO general population cohort, echocardiographic measures, including left ventricular diastolic dysfunction based on age-specific criteria, decrease of systolic deformation of the heart in longitudinal direction (strain) and cardiac remodelling/hypertrophy, emerged independently associated with fatal and non-fatal cardiovascular outcome.2 A combination of these echocardiographic criteria was complementary to predict outcome in 791 randomly recruited subjects.2 Therefore, the assessment of cardiac function and structure using these simple echocardiographic indexes would be important for risk stratification of patients at risk for development of cardiovascular event.
On the other hand, a more comprehensive look at the cardiac images is required to complement indicative trends on large populations and better understand individual behaviours. Thus, the amount of information that can be derived from echocardiography, which is relatively affordable as compared to other imaging techniques, is huge, especially in the new era of precise ultrasound characterisation of myocardial mechanics and development of portable echocardiographic devices. However, until now we have not fully explored the potential of such amount of data.
Biomarkers – a ‘window into wellbeing’
The measurements of biomarkers and modelling their interaction for the evaluation of the health status could be considered as the ‘window into the wellbeing’ of many organ systems. In this regard we have already defined several promising candidate organ-specific proteins and biomarkers through previous efforts. For instance, along with other researchers, we have demonstrated that biomarkers of cardiomyocytes stress (NT-proBNP) and of cardiomyocyte injury (high sensitive cardiac troponin) are associated with diastolic dysfunction and myocardial remodelling in the general population.3 In addition, we have shown that cytokines related to inflammasome activation, mainly interleukin‐18, were significantly elevated in subjects with early asymptomatic stages of heart failure.4
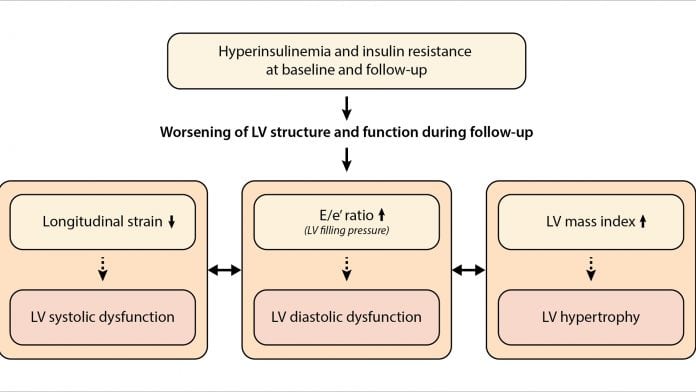
In our recent analysis, we comprehensively assessed the impact of insulin resistance on the natural history of left ventricular remodelling and dysfunction, while considering the complex interrelations between the longitudinal left ventricular changes.5 We showed that higher levels of insulin at baseline and its increase during follow-up were associated with the significant decline in left ventricular systolic performance, worsening of diastolic function and the increase in left ventricular mass index (Fig. 2). Importantly, the observed decline in left ventricular function during follow-up was triggered by insulin resistance even before systemic hyperglycaemia was detected. Interestingly, we observed that in subjects who improved insulin resistance over time, changes in left ventricular function and structure were not significantly different compared with subjects with normal insulin resistance at both visits. Therefore, effective management of insulin resistance along with other risk factors such as hypertension may delay or even prevent the development of adverse left ventricular remodelling and delay the transition to symptomatic heart failure.
Perspectives – towards precision medicine
The cardiovascular area has tremendous potential for personalised medicine and is currently under fast development. In this regard, building integrative computer models utilising the complexity of clinical and behavioural variables, cardiac imaging sequences and pathophysiologically relevant circulating proteins and metabolites for construction of precision cardiovascular classifiers will help to transform the practice of the current state of cardiovascular medicine. The precision computer models will help to create new strategies for risk stratification, prevention, detection and management of early stages of cardiovascular disease, the leading cause of mortality in the community. Moreover, the window of biomarkers should be explored further with regard to their association with heart health status, taking into account the latest high-throughput techniques such as metabolomics and proteomics in the field of biomarkers detection.
References
- Wilkins E, et al. European cardiovascular disease statistics 2017. European Heart Network, Brussels. 2017
- Kuznetsova T, et al. Additive prognostic value of left ventricular systolic dysfunction in a population-based cohort. Circ Cardiovasc Imaging. 2016;9(7)
- Ravassa S, et al. Biomarkers of cardiomyocyte injury and stress identify left atrial and left ventricular remodelling and dysfunction: A population-based study. Int J Cardiol. 2015;185:177-85
- Kuznetsova T, et al. Cytokines profile in hypertensive patients with left ventricular remodeling and dysfunction. J Am Soc Hypertens. 2015;9:975-984
- Cauwenberghs N, et al. Relation of insulin resistance to longitudinal changes in left ventricular structure and function in a general population. J Am Heart Assoc. 2018;7:e008315
Tatiana Kuznetsova, MD, PhD
Research Unit Hypertension and Cardiovascular Epidemiology
KU Leuven Department of Cardiovascular Sciences
University of Leuven
+32 (0) 16345767
tatiana.kouznetsova@kuleuven.be
This is a commercial article that will appear in Health Europa Quarterly issue 6, which will be published in August, 2018.







