Minmin Yen, Founding CEO of PhagePro, illustrates the potential of phages as a solution to antimicrobial resistance.
Antimicrobial resistance (AMR) is a global concern, placing extreme pressure on antibiotics as an effective treatment for a range of infections. It was reported that approximately 700,000 people around the world die of resistant infections every year, due to antimicrobial drugs becoming less effective at killing resistant pathogens.1 By 2050, this number could rise to an estimated 10 million people.2 A recent report by the World Health Organization (WHO) found that none of the 43 antibiotics under development adequately address AMR.
As the situation increases in severity, other solutions are now being developed to tackle the problem of AMR. Biotechnology therapeutics company, PhagePro, aims to use bacteriophages to prevent bacterial infections. Bacteriophages, or phages, are viruses that specifically target and kill bacteria. To find out more about this potential solution to the problem of AMR, Health Europa spoke to Minmin Yen, Founding CEO of PhagePro.
What are the primary goals of PhagePro?
PhagePro is a global health biotech start-up with a mission to reduce AMR in resource-limited settings. By developing strong in-country partnerships, PhagePro’s primary goal is to develop an infrastructure and foundation for successful phage-based solutions that help the world’s most vulnerable communities. With our phages for all approach, our current focus is on prophylactics that can aid in public health campaigns.
How has the misuse and overuse of antibiotics contributed to the spread of drug-resistant infections?
Antibiotic resistance has been reported since antibiotics were first discovered in the 1940s. As humans, we tend to take a silver bullet approach in hopes that an easy solution can solve a complex problem. This has led to antibiotics being misused and overused in all contexts, whether it be clinical, agricultural, or aquacultural. We overly rely on antibiotics to be a solution for everything which has caused an immense selective pressure on bacteria to evolve multiple mechanisms to survive in certain conditions. Now, they are not only surviving, but antibiotic-resistant bacteria are thriving.
What are the primary challenges currently facing the management and treatment of drug-resistant infections?
Funding is the biggest challenge. Especially in the US where our healthcare system is largely privatised, profit drives most of the decisions pharmaceutical companies make about what drugs they will develop.
Many stay away from antimicrobials because companies cannot make money off of them when they are on the market.
In the past few years, we have seen the collapse of two antibiotic companies, Achaogen and Melinta Therapeutics, that produced drugs that worked well against antibiotic-resistant pathogens. There have been a lot of push mechanisms to fund drug development, but there are not enough pull mechanisms on the other side when the product hits the market to keep the companies afloat. Without policy changes, there is little financial incentive to develop antimicrobial drugs, and thus, it will be increasingly difficult to manage and treat drug-resistant infections without more solutions.
Can you explain a little about bacteriophages and how these work to prevent bacterial infections?
Bacteriophages, or phages, are viruses that specifically infect and kill bacteria only. They are naturally found everywhere; there are approximately 1031 number of phages in the world. There are multiple ways that phages can be leveraged to prevent and treat bacterial infections. The primary one is taking advantage of the phage’s natural infection life cycle, to kill bacteria quickly upon contact. Some companies are taking it a step further and engineering phages to enhance them in different ways, such as increasing their host range.
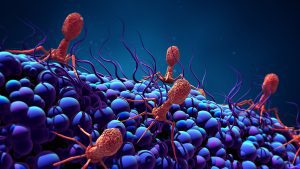
The specificity of phages is a double-edged sword since their narrow spectrum allows for much more nuanced and targeted killing of pathogens, leaving the good bacteria intact. However, it also means that sometimes it can require a number of different phages, or a cocktail, to prevent or clear a bacterial infection. This can complicate logistics in terms of manufacturing, regulatory approvals, formulation, etc., so some companies are expanding the host range of their selected phages through genetic engineering.
How important is cross-border collaboration in supporting AMR research and treatment?
It is the only way we will solve this. As we have seen from the COVID-19 pandemic, our world is quite small, and pathogens do not recognise human-made borders. AMR is a global problem and will require global collaboration. It disproportionately affects resource-limited settings in Africa and Asia, so it is critical that we are collaborating and partnering with in-country scientists and policymakers to identify and develop solutions.
References
- www.unep.org/explore-topics/chemicals-waste/what-we-do/emerging-issues/antimicrobial-resistance-global-threat
- www.pharmaceutical-technology.com/digital-disruption/ai/antibiotic-resistance-ai-tackle-superbug-threat/
Minmin Yen
Founding CEO
PhagePro
www.phageproinc.com
https://www.linkedin.com/in/minmin-yen/
https://m.facebook.com/Phage-Pro-732820107098977/?__tn__=%7E%7E-R
https://twitter.com/phagepro
This article is from issue 20 of Health Europa Quarterly. Click here to get your free subscription today.










