The University of Genoa uses mesenchymal stem cells from bone marrow to treat neurodegenerative diseases. Regenerative medicine represents a challenging area facing one of the major unmet needs of individuals with neurological diseases, namely the repair of neural networks damaged upon injury and resulting in irreversible disability. Recent advances in our understanding of stem cell biology have led to innovative techniques that allow stem cells to be obtained on a large scale and tracked upon in vivo administration.
This, together with the increasing demand from patients for tissue repair strategies, has launched stem cell treatment as one of the most exciting and difficult challenges in the field of neurodegenerative diseases. However, current failures of pharmacological and cellular strategies aimed at repairing the central nervous system reflect the lack of evidence of true tissue regeneration when the neural network is irreversibly damaged.
Thus, strategies should focus on promoting tissue repair before the irreversible loss of neurons has occurred.
The University of Genoa’s expertise
My group at the Centre of Excellence for Biomedical Research (CEBR), and at the Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI), University of Genoa, Italy, has demonstrated that the adult stem/progenitor cells from bone marrow (BM), referred to as mesenchymal stem cells or multipotent mesenchymal stromal cells (MSC) (Fig. 1a), exhibit a significant therapeutic plasticity as reflected in their ability to enhance tissue repair and influence the immune response both in vitro and in vivo.
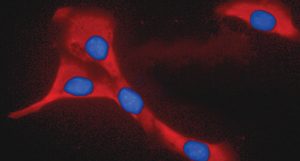
Pivotal experiments have been carried out in mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS), demonstraing that MSC administration inhibits the pathogenic immune response against myelin and neural proteins, protect neurons from damage, and promote remyelination from endogenous precursor cells. Similar results have been obtained by us and other groups following MSC administration in experimental models of stroke, amyotrophic lateral sclerosis and Alzheimer’s disease. Unexpectedly, recent investigations have emphasised that MSC secrete several factors, components of the so-called ‘secretome’, which may suffice to recapitulate many of the effects carried out by these cells on immune and tissue resident cells, thus suggesting that even engraftment in the target organ could be dispensable.
 Upon local or systemic administration, MSC display negligible capacity of trans-differentiation, namely differentiation into neural cells. Nevertheless, they can affect neural cells through cross-talk mechanisms. Importantly, we have demonstrated that inflammatory signals lead to molecular and functional changes, which result in the progressive loss of ‘stemness’ while maximally enhancing their capacity to adapt their therapeutic functions. This new paradigm predicts MSC clinical translation is based on their ability to work mainly as drug stores, delivering therapeutic factors in damaged organs upon interaction with the host environment. It also translates into the need to treat patients with neurological diseases early enough before irreversible damage has occurred.
Upon local or systemic administration, MSC display negligible capacity of trans-differentiation, namely differentiation into neural cells. Nevertheless, they can affect neural cells through cross-talk mechanisms. Importantly, we have demonstrated that inflammatory signals lead to molecular and functional changes, which result in the progressive loss of ‘stemness’ while maximally enhancing their capacity to adapt their therapeutic functions. This new paradigm predicts MSC clinical translation is based on their ability to work mainly as drug stores, delivering therapeutic factors in damaged organs upon interaction with the host environment. It also translates into the need to treat patients with neurological diseases early enough before irreversible damage has occurred.
Based on these results, several small pilot clinical trials in subjects with neurodegenerative diseases, including MS, have demonstrated that MSC administration is safe, and have provided an early signal of clinical effectiveness.
The current aim of clinicians and scientists interested in the development of MSC-based strategies for the treatment of MS is to have the ultimate demonstration that MSC can inhibit CNS inflammation and foster tissue repair as measured, clinically, by functional recovery, or visualised by magnetic resonance imaging (MRI).
With the support of the Italian MS Foundation, a phase II, proof-of-concept, international, double blind, multicentre, clinical trial (MEsenchymal StEm cells for Multiple Sclerosis – MESEMS) with autologous MSC for MS therapy is currently ongoing and aims to answer these questions. If results meet our expectations, the next stage will be to start a phase III multinational clinical trial with the support of patients’ agencies and European funding bodies.
The brain regulates the immune system via mesenchymal stem cells in the bone marrow
While several clinical trials, many of them funded by actions from the European Community, move quickly toward the clinical use of MSC for many human diseases, their biological role in the BM is still only partially understood. Early studies from our group demonstrated through the analysis of the global transcriptional profile of BM-derived MSC that their molecular signature reflects their functional role in the haematopoietic stem cell niche, the anatomical and functional unit in the BM where blood cells are generated. The signature also supports the pivotal role of MSC in the maintenance of the niche homeostasis and the control of haematopoiesis. These early results were complemented by Dr Simón Méndez-Ferrer, who, within the framework of the Marie Skłodowska-Curie Action (‘Career Integration grants’), demonstrated the existence of a connection between the BM and the brain.
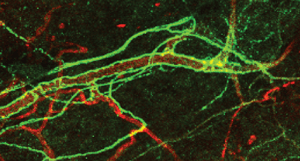
Thus, the brain controls the behaviour of blood stem cells through the innervation of MSC by sympathetic nervous system (SNS) fibres, and results in the control of haematopoiesis via release of norepinephrine (Fig. 1b). Dr David Scadden of the Harvard Stem Cell Institute in Boston, US, recently demonstrated that MSC-derived pivotal molecular drivers enforce the generation of lymphocytes, which are key cells participating in the generation of the adaptive immune repertoire through the interaction of specific MSC populations with haematopoietic stem or lineage-committed blood progenitor cells.
These results led us to hypothesise that the nervous system controls the haematopoietic niche and the generation of the immune repertoire, in an integrated and sophisticated manner, and that MSC play a key role in such a phenomenon.
In a project funded by the Cariplo Foundation (N. 2015 – 1057), Italy, we are currently connecting these dots in the attempt to demonstrate that inflammatory cues sensed by the injured brain lead to the activation of neural sympathetic fibres which, in turn, signal to MSC in the BM. SNS-driven molecular changes in discrete MSC populations drive haematopoiesis and the release in the blood stream of lineage-committed cells, which contribute to modelling specific immune repertoires, possibly relevant for disease pathogenesis.
These studies may provide a new scenario where environmental factors leading to neurological diseases not only shape the immune response through direct interaction with immune cells in the blood and lymphoid organs, but also through the activation of discrete neural populations capable of driving haematopoiesis and favouring the preferential generation of relevant haematopoietic lineage-committed progenitors. Dissection of the mechanisms controlling these events, at the molecular level, are of paramount importance to understand how the brain, upon injury, controls the generation of blood cells, which is relevant for the immune response in homeostasis and pathology.
Finally, I predict that pharmacological or genetic modulation of the activity of these neural populations may be potentially utilised for inhibiting detrimental (or favouring beneficial) immune responses of crucial importance for human diseases.