
Discover how GreenLight Pharmaceuticals Ltd is aiming to advance clinical research, access and education regarding medical cannabis in Europe.
Established in 2014, GreenLight Pharmaceuticals Ltd is an Irish biopharmaceutical company focused on developing safe and effective plant-based medicines. GreenLight specialises in phytocannabinoid research and clinical development. The company is based in Dublin but has recently created a UK based subsidiary and has plans to expand its world of medical cannabis in Europe.
‘Medical cannabis’ (cannabis based medical products (CBMP)) refers to a range of products that contain active compounds (primarily Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD)), either synthesised chemically, isolated from plant, or raw plant preparations.
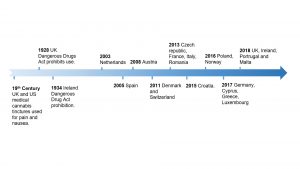
In the past decade Germany, Italy, France, Spain and Switzerland have implemented medical cannabis access programmes for particular indications (Fig. 1). In 2018, Ireland and UK also permitted medical use, however the prescribing process is still nascent, restricting access and uptake. This article briefly explores the current clinical evidence, issues in the UK and Irish patient access and medical education programmes, with GreenLight’s plans highlighted.
Clinical Evidence for medical cannabis use
Evidence of CBMPs clinical efficacy comes from systematic reviews of randomised controlled trials (RCTs). Albeit ‘moderate’ in nature, the strongest evidence emerges from clinical trials in multiple sclerosis, pain and epilepsy. These conditions are consistently cited in government reports from Europe and US as the most appropriate to target with medical cannabis. ‘Moderate’ evidence, as referred to in the definitive 2017 NASEM report, describes ‘several supportive findings from good to fair quality studies with few or no credible opposing findings’.
Multiple Sclerosis (MS)
Standardised cannabis plant extracts of THC and CBD delivered in equal quantities (nabixomols) were the first CBMP to be licensed in the UK for the treatment of MS-related muscle spasm and neuropathic pain, when there is inadequate response to other treatments.
MS patients receiving nabiximols in addition to existing treatment report less muscle spasticity than those receiving a placebo. In 2014, nabiximols were licensed in Ireland, however the Health Service Executive do not currently deem it to be cost-effective.
Chronic pain
Chronic pain, including neuropathic and musculoskeletal pain, is the major reason that patients seek medical cannabis in the US. A 2018 Cochrane review of 14 trials by Mucke and colleagues, reported that a modest but significant increase in the proportion of patients who achieved a 30% reduction in pain; 39% of cannabinoid treated versus 33% who had placebo.
So, while evidence of cannabinoid efficacy against pain remains modest, the proportion receiving benefit remains significant. GreenLight is actively optimising clinical trial designs to find optimal dosing or cannabinoid combinations specifically for pain in arthritis.
Epilepsy
A 2018 systematic review by Stockings and colleagues, summarising RCTs of intractable epilepsy, concludes that adding CBD to conventional anti-epileptic drugs significantly reduces seizure frequency. GreenLight plan to conduct dose finding trials to minimise interactions with co-medications and adverse reactions.
The largest CBD study conducted so far was an open-label study in 261 severe epilepsy patients (average age of participants was 11 years old), who were unresponsive to standard medication. After three months of combined treatment (CBD together with their regular medication), a median reduction of seizure frequency of 45% was observed.
The ‘moderate’ state of evidence clearly indicates limitations which need to be addressed by larger, longer follow-up clinical trials testing cannabinoid combinations, dosage and interactions with other medications. GreenLight are already screening the efficacy of lesser studied cannabinoids from their cultivation programme in a range of disease models prior to clinical trials.
GreenLight currently has eight therapeutic research pipelines based across three core disease areas:
- Neurological conditions including Alzheimer’s, addiction and pain;
- Inflammatory conditions including arthritis, arthritis-related depression, eye disease and diabetes; and
- Cancer including prostate.
The company is currently planning phase II clinical trials of medical cannabis in both arthritic pain and epilepsy.
Access programmes and medical education
A range of medical cannabis regulatory frameworks and provision models are currently employed across Europe, with varying degrees of accessibility for patients. UK and Ireland only recently passed medical cannabis legislature and have some way to go before they operate as effectively as longer standing programmes seen in Netherlands and Germany (Fig. 2).
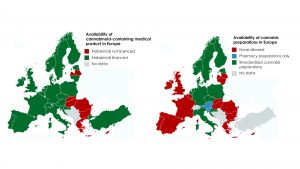
GreenLight Pharma is engaging with regulators in UK, Ireland and beyond to further define and improve patient access programmes. The current frameworks of each constituency are briefly contrasted below.
United Kingdom
In November 2018, medical cannabis was moved from schedule one (no medicinal value) to schedule two, allowing specialist doctors to prescribe for any condition, and for clinical research to be conducted. Since the reschedule, there have been no NHS medical cannabis prescriptions and only 18 in the private sector. Nabiximols are licenced for MS treatment, however standardised plant preparations have not been formally licensed. Doctors are cautious and/or resistant to prescribe for a multitude of reasons including the modest evidence base, hospital trusts refusal to pay and no NICE prescribing guidance (due October 2019). Importantly, GreenLight’s clinical trials will evaluate longer term safety alongside clinical efficacy and effectiveness of CBMPs.
Ireland
In 2017 a HPRA report recommended that medical cannabis should be prescribed in a limited number of medical conditions, where prior treatment has failed, and modest efficacy evidence exists. Specified conditions include spasticity in MS, intractable cancer nausea and severe refractory epilepsy. At the time of writing cannabis-based products containing any THC remain schedule one controlled drugs. The access programme for medical cannabis is embryonic and currently limited in uptake. Prescribing doctors need to secure a licence for named patients from the Minster for Health. Irish supplies of medical cannabis have only recently been secured, with a small number of approved patients permitted to use cannabis products from the Netherlands.
Netherlands
Since 2003, any doctor in Netherlands can prescribe nabiximols or herbal cannabis preparations to treat symptoms of MS, cancer, pain and HIV when patients are unresponsive to, or experience too many side effects from standard treatments. Five medical cannabis preparations from plants with various THC and CBD levels are produced by a licenced company in granulated and raw plant forms. Prevalence of all forms of medical cannabis use including oils reached 24.6 per 100,000 in 2016, primarily for pain.
Germany
Germany has followed a similar legal framework with the creation of the 2017 Cannabis as Medicine act. Patients with treatment refractory conditions can access nabiximols, dried cannabis plant or standardised extract prepared in pharmacies. Prescription is not limited to specialist doctors, nor for specific indications. Germany has tendered for domestic production of up to 2 metric tons of cannabis per anum to standardise the quality of supplies. GreenLight are developing cultivation licences in several countries in Europe and have secured supplier agreements with other countries export markets to ensure sustainable, highest quality supplies of medical cannabis.
Prescribing guidance needed
Training on CBMPs in the UK is yet to be commissioned by the NHS, but online resources are available from The Academy of Medical Cannabis. In Ireland, the HPRA Medical Cannabis access programme have published detailed draft guidelines on CBMPs including dosage and known drug interactions. Several British and Irish medical profession organisations have developed clinical advice on CBMPs. Complementing these efforts, GreenLight has just compiled an online course on cannabinoid prescribing, which will be rolled out in UK and Ireland in 2019.
It is clear that regulatory frameworks for medical cannabis access remain underdeveloped in the UK and Ireland. However, GreenLight is actively leading improvements in medical training, prescription recommendations, standardised supplies and robust clinical trials to help develop access systems that fit regulatory requirements and cultural norms, while meeting clinician and patient demand for safe and effective medicines.
References
- Pertwee R. G. (2006). Cannabinoid pharmacology: the first 66 years. British journal of pharmacology, 147 Suppl 1(Suppl 1), S163–S171.
- NASEM (2017), The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research, National Academies Press for the National Academies of Sciences Engineering and Medicine, Washington, DC.
- Health Products Regulatory Authority (HPRA; Ireland), Cannabis for Medical Use – A Scientific Review, 2017.
- Department of Health (Ireland), Clinical Guidance on Cannabis for Medical Use, 2018.
- World Health Organization Expert Committee on Drug Dependence, Pre-review: Extracts and tinctures of cannabis, 2018.
- Davies, S.C. “Cannabis Scheduling Review Part 1, The therapeutic and medicinal benefits of Cannabis based products – a review of recent evidence” London: Department of Health and Social Care (DHSC; UK) (2018).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA; 2018), Medical use of cannabis and cannabinoids: questions and answers for policymaking, Publications Office of the European Union, Luxembourg.
- Torres-Moreno, M. C., Papaseit, E., Torrens, M., & Farré, M. (2018). Assessment of Efficacy and Tolerability of Medicinal Cannabinoids in Patients With Multiple Sclerosis: A Systematic Review and Meta-analysis. JAMA network open, 1(6), e183485.
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018 Mar 7;3:CD012182.
- Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018 Oct;159(10):1932-1954.
- Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, Herkes GK, Farrell M, Degenhardt L. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry. 2018 Jul;89(7):741-753.
- Dumitru, C. A., Sandalcioglu, I. E., & Karsak, M. (2018). Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Frontiers in molecular neuroscience, 11, 159.
David Gibson, PhD
Chief Scientific Officer
david.gibson@greenlightmedicines.com
Tweet @greenlightmeds
https://greenlightmedicines.com
Please note, this article will appear in issue 10 of Health Europa Quarterly, which will be available to read in July 2019.







