Colzyx is developing a new generation of bioactive wound dressing that accelerates healing and combats infections, including those that are multi-drug resistant.
A novel medical device called WOUNDCOM, which uses highly bioactive peptides derived from the human collagen VI molecule, is the latest development from Swedish company, Colzyx. Colzyx’s proprietary effector molecules are used as an innovative surface coating, designed to fight harmful bacteria, and promote accelerated wound healing.
Science and discovery
Collagen VI is a ubiquitous extracellular matrix component that forms extensive microfibrillar networks in most connective tissues. In 2012, we discovered that the collagen VI von Willebrand factor type A-like (vWA) domains of the alpha-3 chain exhibit a broad-spectrum of antimicrobial activity against Gram-positive and Gram-negative bacteria in in vivo skin infections. In silico sequence and structural analysis of VWA domains revealed that the alpha-3 chain contains cationic and amphipathic peptide sequence motifs.
In vitro and in vivo studies show that these peptides exhibited significant antibacterial activity against ‘normal’ and multi-drug resistant microorganisms through physical membrane disruption. Further in vitro and in vivo studies demonstrated that the same collagen VI-derived peptides actively promote rapid wound closure and wound healing. Taken together, during naturally evolved mechanisms, collagen VI is present in skin wounds where it promotes wound healing and efficiently protects the wound from bacterial infections. Our findings pave the way for developing a novel, highly bioactive wound dressing consisting of a combination of a natural collagen I matrix and collagen VI peptides.
Colzyx’s proprietary effector molecules are designed to:
- Mediate accelerated wound healing;
- Prevent persistent wound infections and subsequent biofilm formation;
- Remove existing biofilm and render bacteria susceptible to antimicrobial treatment;
- Mediate antimicrobial effect, including effect against all known multi-drug resistant bacteria;
- Adjust the level of inflammation; and
- Reduce pain.

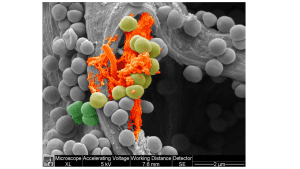
As Colzyx’s effector molecules are derived from human collagen VI, they exhibit a naturally evolved biological activity as compared to inorganic or small molecules. In addition, due to their human origin, Colzyx´s effector molecules are anticipated to have excellent safety and toxicology characteristics.
Colzyx is the first company to utilise molecules derived from human collagen VI for this purpose. The bioactive effector molecules form a developmental platform that can be further applied in Colzyx’s other product development projects, focusing on the coating of bioimplants (BIOCOM project) and on further development as antibiotics (COMBAT project).
Chronic wounds are an increasing global health issue
Most wounds heal but, unfortunately, there are wounds that fail to heal, and such wounds become chronic. Chronic wounds are a significant concern for around 200 million people worldwide and their incidence is increasing with an expected annual growth rate of 3.7% between 2016 and 2023. The dramatic increase in the ageing population will increase these numbers as wound chronicity is associated with age. Other driving factors include the increased prevalence of obesity, diabetes, and cardiovascular disease.
The most common types of chronic wounds are leg ulcers (mainly caused by venous and/or arterial disease), diabetic foot ulcers (caused by vascular and neurological complications of diabetes) and pressure ulcers (caused by unrelieved pressure as a result of immobility). It is estimated that up to 2% of the population will experience a chronic wound during their lifetime. Chronic wounds affect people’s health and quality of life and cause major costs to healthcare systems and the society.
Furthermore, open wounds are an entry point for pathogens making the patient susceptible to severe infections. At the same time, bacterial wound infections are a major driving factor for wound chronicity. Complications of chronic wounds infected with conventional and multi-drug resistant pathogens often lead to lower extremity amputations and sepsis. The rising frequency of chronic wounds, in combination with the global rapid development of multi-drug resistant bacteria, makes this an unfortunate perfect storm.
Multi-drug resistant bacteria are a rapidly growing global health problem
The discovery of penicillin in 1928 was followed by the discovery and extensive commercial production of many other antibiotics. Today we take for granted that any infection with human pathogens is curable by antibiotic therapy. However, the extensive use of antibiotics had a profound impact on the life of bacteria as more strains of bacteria have developed resistance to antibiotics. Some have become resistant to a variety of antibiotics and chemotherapeutic agents, the phenomenon of multidrug resistance.
Indeed, some strains such as methicillin resistant Staphylococcus aureus (MRSA) have become resistant to practically all the commonly available agents. An even more serious threat may be the emergence of Gram-negative pathogens that are resistant to essentially all the available agents.
The development of multi-drug resistant bacteria can be viewed as a silent threat in chronic wounds worldwide. The ever-growing number of antibiotic-resistant bacteria summed to the biofilm present in most chronic wounds, provide a prime environment for horizontal gene transfer and, hence, for nurturing new antibiotic-resistant pathogens. It is vital that the inter-relationships between antibiotic-resistant bacteria and biofilm are interrupted to combat wound infection and chronicity, eliminate the accelerated ‘incubation’ of antibiotic-resistant pathogens, and minimise their environmental spread.
Giving that antibiotic resistance is praised as ‘the greatest threat to human health’ there is pressure to act fast. Indeed, infections with multi-drug resistant bacteria are an increasing global public health threat. In this context, the group of multi-drug resistant bacteria called the ESKAPE pathogens are characterised by their increased resistance to commonly used antibiotics, such as penicillin, vancomycin and carbapenems. In this scenario, chronic wounds can be increasingly difficult to heal, due to infections that cannot be fought using conventional antibiotic treatments. Novel wound care products that address both rapid wound closure and combat infections are urgently needed. The World Health Organization (WHO) described the ESKAPE pathogens as a severe threat to global human health.
Solution
The unique approach in WOUNDCOM is the use of key molecules derived from human collagen VI, a natural host defence molecule present throughout the mammalian body. We are making use of the human body’s own wound healing and antibacterial processes. We have identified, isolated, and further developed key molecules from the human immune system that are produced in the natural wound healing process. The addition of these molecules in the form of WOUNDCOM to a wound has been shown to accelerate the natural wound healing process and synergistically kill bacteria that invade the wound. By this means, WOUNDCOM is able to mirror the body’s own wound healing and rebuilding mechanisms in a new, evolved way.
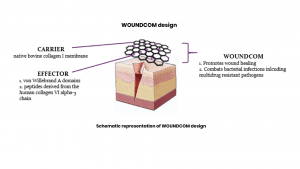
WOUNDCOM has a proven dual function of accelerating wound healing and having a pronounced effect on a wide range of bacterial infections, including a broad spectrum of Gram-negative and Gram-positive bacteria, as well as multi-drug resistant bacteria. Hence, WOUNDCOM represents advanced wound care with specific tissue regeneration properties. The unique approach in WOUNDCOM is the use of molecules derived from human collagen VI and their combination with a biomembrane. Our product acts locally in a wound bed mimicking natural collagen VI upregulation in the healing process and avoids systemic exposure. Our compounds have proved more effective in combating bacterial wound infections and with very limited cytotoxic side effects. The antibacterial properties of WOUNDCOM includes killing of all multidrug resistant bacteria, including the ESKAPE bacterial pathogens as defined by the WHO.
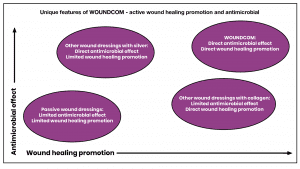
This unique solution creates a new and innovative category of wound care products and addresses a new market segment of interactive biomembranes on the global market. Our solution can be used in acute as well as in chronic wounds. Faster and more efficient treatment of acute and chronic wounds implies several benefits for patients and medical professionals and will increase demand for WOUNDCOM in hospitals and clinics.
In summary, as our invention has defined antibacterial effects against all bacteria tested so far, including multidrug resistant bacteria, it will combat multidrug resistance as listed by the WHO as one of the biggest threats to global health.
How WOUNDCOM compares
Molecules used for wound matrix coating today are based on inorganic or small molecules. These molecules stimulate either wound healing (e.g., bovine collagen-based products), or exert antibacterial effects (e.g., silver-containing products). In contrast, Colzyx wound matrix utilises effector molecules which are bioactive parts from the natural biological host-defence protein, human collagen VI. This collagen has evolved to initiate, stimulate, and control normal physiological wound healing processes as well exhibiting clear antimicrobial effects. Thus, Colzyx effector molecules have both a pronounced wound healing effect and, at the same time, a broad-spectrum antimicrobial effect, against both normal and multidrug resistant bacteria. Thus, as compared to other wound healing products, WOUNDCOM has the potential to substantially reduce costs associated with lengthy healing processes and treatment of acquired infections.
Horizon 2020
In 2019, Colzyx received a total of approximately €5.5m in financial support from the Horizon 2020/EIC Accelerator programme. The support consists of approximately €2.5m in grants and an investment from the European Investment Fund (EIF) of approximately €3m. The investment is part of the recently formulated ‘blended finance’ initiative and Colzyx is one of two Swedish companies that, for the first time, qualified for this type of investment. In May 2020, the Grant agreement was signed between Colzyx and the European Commission.
WOUNDCOM is presently being developed with the aim of being registered as a medical device and will be evaluated in clinical trials focused on the healing of chronic wounds. Colzyx’s preclinical studies are concluded. The biological evaluation of WOUNDCOM will be initiated in autumn 2021. Colzyx’s clinical trials, with the support of competent and experienced clinical partners and PIs, are planned for Q2/Q3 2022. The current plan is to perform this study in dermatology clinics and wound centres in Germany and Sweden. Patients with infected chronic leg ulcers will be enrolled in this study.
Colzyx’s timeline
- 2012: Matthias Mörgelin together with Suado Abdillahi at Lund University discover that Collagen VI exhibits unprecedented wound healing and antimicrobial activity. This work was a starting point for what would become the foundation of the Colzyx.
- 2015: Upon making the Collagen VI discoveries, Matthias decided to contact Eskil Söderlind who he had known for several years, and present the discovery and ideas.
Eskil presented the idea to his business partner Karin Bryder and they decided to further evaluate the possibilities to commercialise the discoveries.
- 2016: Colzyx AB was founded by the two inventors Suado Abdillahi and Matthias Mörgelin together with Karin Bryder and Eskil Söderlind. The first patent application was filed.
- 2018: Key findings were published in Journal of Immunology. Colzyx received grants of total €80,000, from Vinnova and through the Horizon 2020 SME instrument/Phase 1 initiative from the European Commission.
- 2019: A first seed investment was made by the CBC investment group.
Colzyx receives a total of approximately €5.5m in financial support from the Horizon 2020/EIC Accelerator programme.
This article is from issue 19 of Health Europa Quarterly. Click here to get your free subscription today.











