
Belgian company miDiagnostics is developing an ultrafast RT-qPCR COVID-19 test which could accelerate a return to international travel.
The COVID-19 pandemic has highlighted the challenges the diagnostics industry is currently facing. At the start of the pandemic, it was a struggle to cope with the demand for increased testing capacity within an already stretched healthcare system. Now, as we hopefully approach the end of the current pandemic and life starts to return to normal, governments are working hard to implement effective testing in an increasingly vaccinated population.
miDiagnostics is a Belgium-based company currently developing a COVID-19 polymerase chain reaction (PCR) point-of-need test. This test will have a similar performance to the current reference PCR methods used in centralised settings but will only take 15 minutes to get a result. Silicon chip technology is at the heart of this innovation (Fig 1). This highly accurate, fast, and scalable test could play a pivotal role in screening people in decentralised settings, such as in the workplace, at events and in airports (Fig 2). Founded in 2015, miDiagnostics is a spin-off of imec, the world-leading research and innovation hub in nanoelectronics and digital technologies, and underpinned by a research collaboration with Johns Hopkins University, the highly reputed US research and medical centre.




miDiagnostics’ ultrafast, point-of-need PCR test takes 15 minutes from sample to result
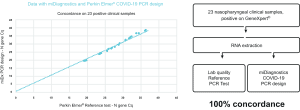
miDiagnostics’ rapid COVID-19 PCR test combines the accuracy of a central, lab-based PCR test with the speed of a rapid antigen test. A nasopharyngeal swab eluate is loaded onto a small testcard, containing a silicon chip which performs the PCR test. Data analysis is completed via a compact and portable reader.
Key benefits of the 15-minute COVID-19 PCR test

Lab quality PCR performance
With the summer of 2021 now well underway and everyone keen to fly abroad for their holidays, miDiagnostics’ CEO, Katleen Verleysen, spoke with experts in different fields to discuss how the pandemic has impacted their work and what is needed to help support a return to normal life in coexistence with COVID-19.
COVID-19 has decimated the global aviation industry
The aviation industry has been particularly affected by the COVID-19 pandemic and many international airports were operating at less than one third of their capacity. The industry is desperate to find ways to recover. The vast majority of airports are offering testing services, in order to ensure safety for their travellers and to help to comply with the strict travel policies dictated by national governments. Due to its high sensitivity, PCR testing is still regarded as the most suitable test to ensure safe travel. Passengers are therefore asked to take a test less than either 48 hours or 72 hours prior to departure, all depending on the country of arrival. Due to the logistics and slow turnaround time of current PCR testing, a test just prior to boarding is not feasible at the moment.
Yet what if PCR testing only took 15 minutes? This would open up avenues towards screening prior to boarding, screening of transit passengers and much more efficient processes for aviation crew.
Bart Saverwyns, Chief Experience Officer of Brussels Airport
How has the aviation/travel industry been impacted by COVID-19?
“COVID-19 has been the biggest challenge to the global aviation and travel industry in living memory. The sector is still reeling from this and is expected to still take three years to return to normality.”
How do you see the future of Brussels Airport and its co-existence with COVID-19?
“As travel picks up again, we are faced with multiple challenges: new COVID-19 variants, different levels of vaccination, the risk of inaccurate test results and differing testing requirements for different countries. Our vision is that testing will remain an integral part of travel procedures at least for some time and most certainly for specific destinations. Therefore, pending global vaccination and in looking for a valid alternative to quarantine requirements, Brussels Airport is looking into the most effective ways to get testing done at the airport, ensuring the comfort and safety of its passengers. Ideally, this means a seamless solution, avoiding crowds gathering and queuing, hence we are looking to create the right infrastructure for the testing needs of the future.”
Would the airport benefit from a fast and accurate test?
“An ultrafast and accurate test would enable testing prior to entering the airport. This means passengers would no longer have to get tested several days before their trip, but instead they could arrive a little earlier at the airport and get tested in the hours before their flight. Furthermore, we are looking into ways of screening transit passengers, to ensure safe travel to their final destination. To enable this, mobile units and ultrafast test turnaround times are needed. In addition, airline crews definitely could benefit from the option of having repeated and efficient testing available at the airport.”
With the summer period ahead, how will you deal with the increasing demand of testing?
“We are working with our partners, Ecolog International and Eurofins, to create solutions that allow flexibility in testing volumes. Last Christmas we saw an increase in passengers and rapidly scaled up our testing capabilities by opening two extra test centres on site, one as a drive-through and one in the arrivals hall. This rapid scaling and flexibility are crucial for us. We are looking at diagnostic solutions that allow this rapid scaling and flexible set-up. Decentralised testing centres are needed to avoid crowds gathering (for transmission and safety purposes), as well as queuing. The management of our passenger flow is one of our priorities in these times.”

The importance of innovation and strong partnerships in COVID-19 diagnostics development
As a spin-off of imec and Johns Hopkins, miDiagnostics has access to some of the world’s most respected scientific institutions. Verleysen asks them about the critical role of innovation, clinical knowledge, sharing knowhow and strong partnerships in COVID-19 diagnostics development.
Luc Van den hove PhD, CEO, imec
Luc Van den hove is President and Chief Executive Officer (CEO) of imec, a role he has held since 2009. Before this, he was Executive Vice President and Chief Operating Officer at imec.
Under his leadership, imec has grown to an organisation with a staff of around 4,000 people, operating with an annual budget of around €640m (2019) and with offices in Belgium, the Netherlands, US, Japan, Taiwan, China and India. Luc joined imec in 1984.
Luc is also Professor of Electrical Engineering at the University of Leuven and a member of the Technology Strategy Committee of ASML.
What is the relationship between imec and miDiagnostics?
“miDiagnostics’ core nanofluidic technology was developed in-house at imec. As a world-leading R&D and innovation hub, imec designed the core ultrafast PCR technology about 10 years ago with the vision to develop PCR tests outside of centralised settings such as hospitals. Once the first prototype chip was developed, imec, together with Johns Hopkins, spun out the technology ensuring its proper commercialisation.”
How important has COVID-19 been to innovation?
“COVID-19 has only strengthened the vision we had 10 years ago to get accurate and scalable diagnostics closer to the point-of-need. Diagnostics has been underrepresented in the last decade and this pandemic has made clear that investing in diagnostics and innovation is of utmost importance. We will have to live side-by-side with COVID-19 for many years to come. Due to the ever-growing and increasingly mobile global population, this will not be the last pandemic. PCR is also the golden standard in RNA/DNA diagnostics and therefore presents a very versatile platform for the future.”
What do you see is the future for COVID-19 detection?
“We are excited by the work of miDiagnostics, who turned the silicon PCR chip into a portable, rapid and lab-quality diagnostic test at the point-of-need. Nasopharyngeal swabs, apart from being very unpleasant, still requires a healthcare professional to take a sample from an individual, so this becomes the limiting factor in massive parallel screening of big crowds. imec has been working on a truly innovative way of sampling, whereby the silicon PCR chip captures aerosols and droplets from exhaled breath, followed by accurate PCR measurement of viral RNA (so not the classical volatile organic compounds (VOCs) testing). miDiagnostics is looking into the compatibility of this technology with their current setup.”
Why do you work in a partnership model with miDiagnostics and others?
“As an R&D centre, we are committed to delivering new ideas and innovation to solve important problems for humanity, but we need partners to take these ideas and convert them into qualified products, taking them through clinical trials, product development, and ultimately towards commercial launch. It has been great to see how miDiagnostics has adapted to changing diagnostics needs, pivoting quickly from its broader research in Complete Blood Count (CBC) and Molecular Diagnostic (MolDx) testing, to a sharper COVID-19 focus.”
Stuart C. Ray, MD, FACP, FIDSA, Johns Hopkins University
Prof Dr Stuart C. Ray is a Professor of Medicine in the Division of Infectious Diseases, Vice Chair of Medicine for Data Integrity and Analytics, co-chairs the Research Subcouncil of the Johns Hopkins Medicine (JHM) Data Trust Council, is a faculty member of the Immunology and Pharmacology graduate programs and directs a virology laboratory where he studies the interplay of viral evolution and human immunology. With his experience in the fields of virology and immunology, he explains how the diagnostics sector has innovated as a result of COVID-19 and what the greatest needs are in the next phase of this pandemic.
How has your field of study changed and innovated as a result of COVID-19?
“At the beginning of the COVID-19 pandemic, the World Health Organization (WHO) urged countries affected by COVID-19 to rapidly scale testing capacity to enable accurate situational assessment and mitigate outbreaks. This is still relevant, although it is critical that these tests are accurate, fast and available at the point of care.
“The emergence of COVID-19, and the detrimental effect it has had on the global economy over the last year, has meant that everyone now takes an active interest in virology, no matter what their profession or level of scientific knowledge. Virology expertise is now in huge demand and of course we are being called on to share insights on how we might be able to return to normal life.”
What are the drawbacks of existing diagnostic solutions? What are the greatest needs and requirements for an effective diagnostic testing strategy?
“Conventional diagnostic solutions are distributed along what seems to be a trade-off between diagnostic accuracy and convenience of speed and portability, rarely providing rapid and clinically accurate results at the point-of-need. It is critical for fast and accurate PCR COVID-19 tests to be available in key locations such as airports, schools and offices. This would help support a safe return to normal life, alongside mass vaccination. Fast PCR tests would have an important role, not just in our control of COVID-19 and its spread, but for future infectious disease outbreaks.”
How can technology play a role in fulfilling these needs and requirements?
“Silicon-based technology has enabled multiple revolutions since its first applications. Medical diagnostic technology has benefited partially, but the trends of miniaturisation and decentralisation are only beginning to innovate the diagnostics sector. Compact, rapid, highly reproducible connected devices hold promise for making accurate diagnostics available to all people, enabling continual rapid improvement in scale and cost.”
What are the crucial characteristics for a diagnostic product?
“A diagnostic product must have a clearly defined intended use and generate accurate results across a relevant target concentration range, working from a specimen that can be collected reproducibly, and provide results rapidly enough to be useful. The product must be consistently manufacturable at a suitable scale and be easy to operate by the personnel available in a relevant setting.”
How does miDiagnostics answer these needs and requirements?
“Built upon biomedical technology expertise and innovation arising from an interplay between imec and Johns Hopkins University, miDiagnostics brings the control and scalability of silicon microfluidics to rapid and clinically relevant diagnostic applications.”
Our vision for the future
miDiagnostics’ vision is to bring fast, affordable, scalable and accurate diagnostics closer to the point-of-need, pharmacy setting and even into the home setting. PCR as a molecular diagnostic tool is still the highest-quality reference point, due to its sensitivity and specificity, as well as its versatility. We believe that taking this technology out of the centralised setting and closer to the patient/consumer is part of the future of healthcare.
We want people to resume all kinds of activities that they took for granted before the COVID-19 pandemic and for life to return to normal. We look forward to sharing progress with you as our COVID-19 test is developed further, approved and made commercially available. The test will also be expanded towards influenza A/B, RSV and other respiratory indications. While the initial roll-out is foreseen for airports, we are also in discussions to employ this innovation for events, conferences, schools, hospitals, pharmacies, and the workplace.
This technology will also be made compatible with other means of sampling such as saliva and aerosols – droplets collected from exhaled breath. The latter innovation, currently being developed at imec, shows great promise and would have a major impact in fighting this and future pandemics.
We truly hope that the next time a pandemic hits, we can be proactive instead of reactive, with numerous countries going into lockdown and the economy coming to a standstill.
Together with our founders, imec and Johns Hopkins, our clinical partners UZ Leuven and beyond, our engineering and supply chain partners, as well as the growing team of talented individuals at miDiagnostics, we are well positioned to make a major impact and help shape the future of healthcare.
miDiagnostics has never felt so relevant, timely and critical; and I feel privileged to be leading the company in these challenging times.
This article is from issue 18 of Health Europa. Click here to get your free subscription today.











