Expanding the boundaries of medical cannabis clinical evidence; removing barriers to medical cannabis practice.
In this article, Amit Sade, of Sade Group, discusses expanding medical cannabis clinical evidence and removing barriers to practice.
Science and technology are essential tools for innovation. Harmoniously engaged, they play a crucial role in bettering quality of life, sustaining health and prolonging longevity. We live an era where emerging technologies have profound impact on almost every industry; thus, key players need to refocus their strategies in order to stay relevant and thrive.
Alongside these challenges, and amid the current COVID-19 pandemic, the healthcare ecosystem is facing exciting opportunities to transform vision into reality for the benefit of patients, healthcare providers and society as a whole.
New approaches to medical development
For the past half century, medical developments have relied on clinical trials to demonstrate safety and efficacy.
Ubiquitously referred to as the ‘gold standard’ of clinical investigation,1 randomised controlled trials (RCTs) have long been viewed as the topmost level of scientific evidence for determining whether specific medical interventions work. Accordingly, patient care has been dominated by evidence-based medicine with its emphasis on RCTs and clinical guidelines to standardise medical decision making.2
Nonetheless, drug development has been a costly and lengthy process with an extremely low success rate and lack of consideration of individual diversity.3 Traditional RCTs may not provide sufficient information on how well the drug works under real world conditions in varied contexts (eg polypharmacy or comorbidities) and across patient subpopulations.4
The alternative ‘Big Data’ approach has been expanding at an exceptional pace based on the development of highly advanced electronic databases, machine learning and native language processing, along with the growing emphasis on treatments tailored to the individual and the need to shift the focus from evidence-based medicine to medicine-based evidence.
The hope and hype of real world data
When used in the healthcare context, the term ‘real world data’ (RWD) usually refers to patient-level data gathered outside the conventional clinical trial setting. Such data may be generated in the course of normal clinical practice or it may be reported directly by patients.4 Clinical evidence that is derived from the analysis of RWD is then considered as real-world evidence (RWE).
Technological evolution, alongside increased pressure from regulators, payers, prescribers and patients have resulted in:
- The ability to capture RWD from a variety of resources – web searches, mobile devices, wearables, at-home genetic kits, social media platforms and online patient communities; and
- Competence to analyse the data proliferation to generate meaningful insights.
Evidently, RWD has been gaining attention across the community; and is expected to expand as a result of the various initiatives and efforts carried out in the sector.5
Can real world data support the practice of medical cannabis?
Medical cannabis is a revolutionary product that is garnering mass acceptance globally. It has the potential to create a huge impact on the healthcare industry to cure cases that are not treatable by traditional medicines.6 A recent study in Canada even demonstrated that certain strains of cannabis can inhibit viral activity caused by the novel coronavirus.7
A comprehensive review of over 10,000 recent studies conducted and published by the National Academies of Sciences, Engineering, and Medicine (NASEM) concluded that there is ‘conclusive and substantial evidence’ that pharmaceutical grade cannabis is effective for alleviating chronic pain, chemotherapy-induced nausea and vomiting, and spasticity associated with multiple sclerosis.8
Despite the myriad of studies that have examined cannabis use in all its various forms, it is still claimed that conclusive evidence regarding the short and long-term health effects of cannabis use remains elusive.8 The inevitable conclusion is that solely relying on the evidence-based approach when examining scientific research into cannabis may not be sufficient to fully comprehend the therapeutic value of medical cannabis data. Legalities and evidence-based recommendations aside, the reality is that many patients seek out medicinal cannabis, either as a substitute or complement to standard medications.
Physicians and patients’ perception and knowledge about medical cannabis
Findings from one of the largest surveys of medical cannabis published to date9 demonstrated that patient-reported outcomes favour strong efficacy for a broad range of medical conditions (pain, anxiety, depression, migraine and muscle spasticity). Participants reported an 86% reduction in symptoms a result of cannabis use, with nearly 60% of medical users reporting substituting cannabis for prescription medications.
A survey of primary care providers10 revealed that:
- A majority of healthcare providers (58.1%) believe that medical cannabis is a legitimate medical therapy and believe that providers should be offering it to patients; and
- One half of providers were not ready to answer questions about medical cannabis, and wanted to learn more about it.
These findings suggest critical gaps in research, medical education and policy regarding medical cannabis. There seems to be a clear discrepancy between policy and implementation; and a noticeable need for cannabis education.
Realising the promise of medical cannabis through a novel shared technology platform
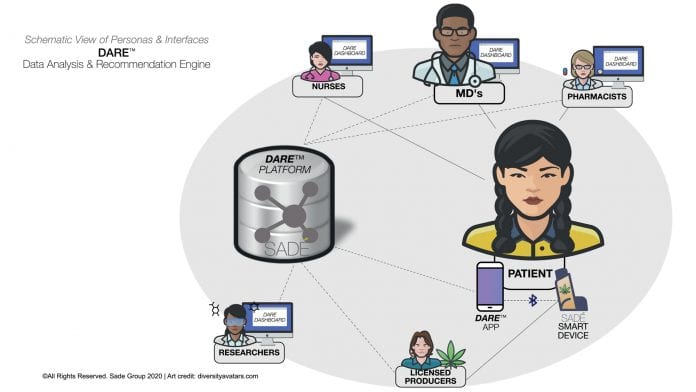
Determined to bridge the knowledge and evidence gap, Israel-based Sade Group is developing a novel Big Data platform which will constitute a shared technology base for the entire ecosystem of medical cannabis.
Integrating real-world health data with human and machine intelligence, Sade’s hybrid healthcare solution (see Fig. 1) will provide the much needed evidence to manage individual patients while enhancing professional engagement and improving treatment retention rates.
“Harnessing our experience, talent and passion, we’ve embarked on a quest to turn patient centeredness from rhetoric to real practice. Realising the immense potential of medical cannabis requires the entire industry to work together to finally attain the guidance and evidence sorely needed to manage individual patients,” said Yaron Gissin, Chief Innovation Officer of Sade Group.
Founded by a team of highly experienced life science executives and cannabis breeders, Sade is focused on realising the promise of medical cannabis through key activities including:
- A Data Analysis and Recommendation Engine (DARE™): a real time healthcare network built upon human and machine learning to power better care for patients;
- The development of a smart, connected medical device – a unique mechanism enabling the administration of a precise, consistent dose of medical cannabis oil, along with improving its stability and quality;
- Cultivating medical grade cannabis varieties, through advanced agriculture and breeding technologies, in its huge state of the art indoor hydroponic facility in Macedonia; and
- Partnerships and investment in plant genetics mapping, analysis and clinical research.
“We dare to share. By creating a consortium of medical cannabis stakeholders that is continuously exchanging knowledge and experience, our unique Big Data platform will constitute a huge database of real-world evidence. Tapping into the vast amounts of validated data may enable to provide people with treatments that are based on the best available evidence,” said Amit Sade, CEO of Sade.
Sade is calling on companies, researchers and individuals to join this emerging vibrant ecosystem. No matter what perspective you may bring – patient associations, health organisations, caregivers, local producers – we’d like to hear from you.
References
1 David S Jones at al. The history and fate of the gold standard. The LancetVol. 385, ISSUE 9977: 1502-1503, APRIL 18, 2015
2 Ralph I. Horwitz et al. Medicine based evidence and personalised care of patients. Eur J Clin Invest.2018;48:e2945.
3 Rosa S. Kim et al. Use of big data in drug development for precision medicine. Expert Rev Precis Med Drug Dev 2016; 1(3): 245–253.
4 Brandon Swift et al. Innovation at the Intersection of Clinical Trials and Real-World Data Science to Advance Patient Care. Clinical and Translational Science (2018) 00, 1–11 Pharmacoepidemiol Drug Saf. 2019;1–12
5 Winona R. Bolislis et al. Use of Real-world Data for New Drug Applications and Line Extensions. Clinical Therapeutics/Volume 42, Number 5, 2020
6 ‘Medical Marijuana Market by Product, by Application and by Distribution Channel : Global Industry Perspective, Comprehensive Analysis, and Forecast, 2017 – 2024’. Report at https://www.zionmarketresearch.com/report/medical-marijuana-market
7 Bo Wang et al. University of Lethbridge: In Search of Preventative Strategies: Novel Anti- Inflammatory High-CBD Cannabis Sativa Extracts Modulate ACE2 Expression in COVID-19 Gateway Tissues. ResearchGate Publication, posted April 2020
8 National Academies of Sciences Engineering and Medicine (US) Committee on the health effects of marijuana: an evidence review and research agenda: the health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: National Academies Press; 2017available at http://nap.edu/24625
9 Sexton, et al.: A Cross-Sectional Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy Cannabis and Cannabinoid Research 2016, 1.1. http://online.liebertpub.com/doi/10.1089/can.2016.0007
10 Lindsey M. Philpot et al. A survey of the attitudes, beliefs and knowledge about medical cannabis among primary care providers. BMC Fam Pract. 2019; 20: 17.
Amit Sade
Sade Precise Agriculture
amits@sade.group
www.sade.group
This article is for issue 3 of Medical Cannabis Network. Click here to get your free subscription today.









