Infection prevention specialists, GAMA Healthcare, discuss the burden of drain-based biofilms and how these can be tackled in healthcare.
Are we throwing our best antibiotics down the drain? Outbreaks of drug-resistant organisms are increasingly linked to contaminated hospital sinks and shower drains, and the biofilms lurking down the plughole. Unfortunately for us, current techniques are not working.
In 2011, at Seoul National University Children’s Hospital in South Korea a paediatric ICU struggled against an outbreak of Acetobacter baumannii – a species of Gram-negative bacteria infamous for developing resistance to multiple antibiotics at once. With immunocompromised patients on the ICU and limited treatment options, infections can prove fatal.
For several months, the Infection Prevention and Control (IPC) team battled to resolve the outbreak. They elevated regular surface disinfection, cohorted the nurses working on the affected ward, and carried out active surveillance and microbiological sampling.
With the test results, they navigate the map of positive samples back to one particular sink. The sink – used for hand washing – appeared to be contaminating the surrounding environment due to splashback.
Despite disinfecting the sink in question five times per day with sodium hypochlorite, the outbreak persisted. Eventually the sink in question was replaced and the outbreak was resolved within a month.1
But the outbreak in Seoul is not unique. An 860-bed university hospital in France described a similar outbreak of A. baumannii, traced back to their sinks.2 Likewise, sinks were responsible for an outbreak of Klebsiella pneumoniae in Spain.3 In both cases, the concentration of sodium hypochlorite used was ineffective.
Biofilms – the hidden cause of persistent outbreaks
We tend to think of microorganisms as free-floating, a collection of individual organisms. This assumption is so ingrained that most disinfectant efficacy testing is done on these planktonic organisms. Unfortunately for us, that is not always how microbes behave in the real world.
When microbes attach to a surface, they can form protective biofilms. Different microbial species work together to form a protective matrix that protects them from traditional disinfection.4–6 With multiple species sheltering within the same biofilm, microorganisms are able to pass antibiotic-resistance genes from one species to another – a process known as horizontal gene transfer. When we allow biofilms to form, microbes become much harder to kill, both with disinfectants and antibiotics.
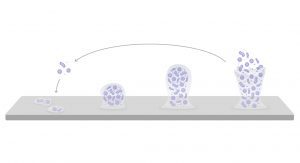
How do we stop biofilm-fuelled outbreaks?
If chlorine-based disinfectants cannot stop outbreaks, does that mean we all need to regularly change our sinks? A group of researchers at Cardiff University in the UK set about trying to understand why regular disinfection with sodium hypochlorite had not worked in those previously mentioned outbreaks.7
They set to developing a model of the two most common hospital drain types: U-bends and drainage traps. Within those models they began growing complex, multi-species biofilms. From there they were able to test the efficacy of various disinfectants against drain-based biofilms. What they found was a surprising insight.
Whilst the chlorine-based disinfectants (sodium hypochlorite and NaDCC) were able to reduce viable organisms in the top-most part of the drains, both chlorine-based disinfectants were unable to produce any significant reduction in the middle or lower areas of the drain.
They also found that, when treated with chlorine-based disinfectants, the biofilms were able to rapidly recover – suggesting the unaffected biofilm in the lower parts of the drain provided a ‘life raft’ for the microbes to rapidly recover.
Those findings are not surprising: chlorine-based disinfectants are inactivated by organic matter (and biofilm matrixes are composed largely of dense organic matter).8 Clearly, if we are going to put a plug in drain-based outbreaks, we need a more effective alternative.
Peracetic acid-generating technology
Peracetic acid is a powerful oxidative disinfectant. Unlike chlorine, it is not inactivated by organic matter – making it a great candidate for tackling drain-based biofilms. The biggest challenge is stability: peracetic acid has a very short shelf life unless kept at a very low pH. Many generic peracetic acid formulations are highly acidic and corrosive.

Clinell Drain Disinfectant is developed using patented technology from Clinell Peracetic Acid Wipes. Rather than relying on keeping liquid peracetic acid at a low pH, Clinell Drain Disinfectant only generates peracetic acid when exposed to water.
Generating peracetic acid only when it is needed, Clinell Drain Disinfectant works at a near-neutral pH. It has been tested against common drain materials to avoid unnecessary, costly damage to sinks and drains.
When tested by investigators in Cardiff, they found that only Clinell Drain Disinfectant was able to eradicate the biofilm throughout the drainage trap.7 Additionally, they found Clinell Drain Disinfectant prevented biofilm regrowth for at least four days – the only product tested to do so.
Tapping the potential
Drain-based biofilms trap hospitals into a cycle of continuous outbreaks. Traditional, low-cost chlorine-based disinfectants are not designed to tackle drain-based biofilms, they are not working, nor are they saving anyone any money. Especially if hospital hardware is replaced each time there is an outbreak. If we are going to fight back against biofilms, we need more effective, more suitable, easier to use alternatives.
One sachet of Clinell Drain Disinfectant, combined with running water, generates a powerful blend of oxidative agents. When used as directed, they form an easy drain disinfection protocol, that breaks down the biofilm matrix and kills the microorganisms sheltering inside.
To find out more, speak to your local GAMA Healthcare Area Manager, or visit www.gamahealthcare.com
References
- Hong et al. Pediatr Infect Dis J. 2012;31(7):685-690.
- Landelle et al. Infect Control Hosp Epidemiol. 2013;34(2):119-124.
- Seara et al. Int J Antimicrob Agents. 2015;46(2):169-173.
- Otter et al. J Hosp Infect. 2015.
- Ledwoch et al. J Hosp Infect. 2018;100(3):e47-e56.
- Ledwoch & Maillard. Materials (Basel). 2019;12(1):4-13.
- Ledwoch et al. J Hosp Infect. 2020;106(4):757-764.
- Humphreys et al. J Infect Prev. 2013;14(4):126-131.
This article is from issue 20 of Health Europa Quarterly. Click here to get your free subscription today.











