There have been more than 7,500 approvals for medicinal cannabis in Australia since legalisation in 2016 – so why don’t we know if it is helping patients?
Access to medicinal cannabis for patients was legalised in Australia in 2016, allowing doctors to request permission to prescribe from the Australian government. Prescription occurs mostly on a case-by-case basis involving evidence that patients have failed to benefit from available conventional prescription medications. Since legalisation, a few thousand Australians have been prescribed a medicinal cannabis product.
Despite this, many tens of thousands of Australians continue to self-medicate with illicit medicinal cannabis. This is at least due to the difficulty and cost involved in accessing a legal prescription. Additionally, doctors generally do not feel well-educated around the use of medicinal cannabis products, with specialists often feeling that there is inadequate evidence to support their use in specific conditions.
The Therapeutic Goods Administration (TGA), Australia’s medicines regulatory body (akin to the FDA in the USA and EMA in Europe), is currently collecting some potentially useful information from doctors prescribing medicinal cannabis around the progress of their patients. However, the TGA does not appear to be analysing or disseminating this information for use by health professionals or the patient community. Here we explore how the Australian government might be missing a key opportunity for research into medicinal cannabis and what might be done to address this.
Patient access pathways
First, we will explain how Australians obtain a medicinal cannabis prescription. Australia has chosen to incorporate medicinal cannabis into our existing health regulatory framework governing unregistered medicines. In Australia, drugs that doctors prescribe conventionally have obtained ‘registration’ with the TGA. To obtain registration, a drug sponsor is generally required to run large-scale clinical trials proving safety and efficacy and provide the resulting evidence to the TGA. With one exception (Nabiximols, aka ‘Sativex’), this process has not been completed for any medicinal cannabis products in Australia. This means that the more than 50 other medicinal cannabis products in Australia, including oils, capsules, sprays and botanical material, are ‘unregistered’ medicines.
There are two main ways Australian patients gain access to unregistered medicines: The Authorised Prescriber Scheme and the Special Access Scheme Category B (SAS-B). So far, around 60 Australian doctors have been granted Authorised Prescriber status for a cannabis medicine, meaning they can freely prescribe to a specific class of patients directly under their care. Becoming an Authorised Prescriber is an onerous, time-consuming process and restricts the prescriber to one medicinal cannabis product and one patient class. The most common example of Authorised Prescribers are paediatric neurologists prescribing the FDA-approved cannabidiol product Epidiolex to children with refractory epilepsy under compassionate access schemes in New South Wales and Queensland.
Authorised Prescribers are only required to notify the TGA within 6 months of prescribing an unregistered medicine to a patient. So accurately determining how many people are currently accessing medicinal cannabis through this scheme is difficult to ascertain, although it has been estimated to be between 20% and 30% of Australian medicinal cannabis patients.
The remainder of patients receive access to products via the SAS-B pathway, which allows doctors to request a prescription for an unregistered medicine for a specific patient on a case by case basis. Approval is granted by the TGA but may sometimes require additional State/Territory Health department approval depending upon patient location and product choice.
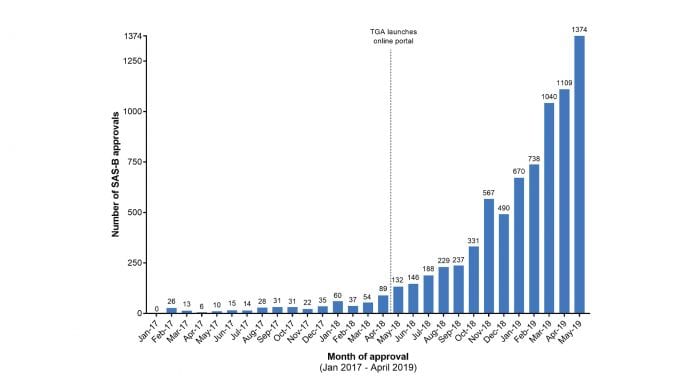
The number of individual approvals through the SAS-B pathway has increased from a handful per month in early 2018 to over one thousand per month as of April 2019 (see Fig 1). Each approval represents a prescription authorisation for an individual patient, usually for an amount of medication that will last between one and twelve months.
Clinical evidence gaps
Despite the substantial rise in monthly approvals, overall patient numbers are small. This is at least partly due to the unresolved questions about the efficacy of medicinal cannabis in treating many conditions. Recently published reviews indicate modest to good quality evidence to support cannabis use in chronic non-cancer pain, multiple sclerosis spasticity, paediatric epilepsy, palliative care and chemotherapy induced nausea and vomiting (CINV).
Summarised critical reviews of the existing clinical evidence for medicinal cannabis use in each of these five areas has been compiled by the TGA and comprises their ‘clinical guidance’ documents, which are publicly available. However, these documents have not been updated since their creation in December 2017, which, given the recent acceleration of clinical research into medicinal cannabis, means clinicians are not always being guided by up to date summaries, and are left to do their own research in many areas.
Unsurprisingly, the indications with the highest quality of positive published evidence, and most supported by the TGA guidance documents, account for the majority of SAS-B approvals. Fig 2 shows a breakdown of the SAS-B approvals from January 2018 To February 2019 showing a general upwards trend heavily skewed toward prescriptions for chronic non-cancer pain. (We should note that condition-specific data is only available up to February 2019 and we were granted access to these data only after a formal Freedom of Information Request was approved in April 2019).
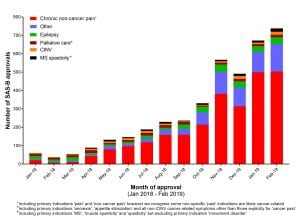
There are a range of conditions where the clinical evidence for medicinal cannabis efficacy is minimal to non-existent (shows as ‘Other’ in Fig 2), yet doctors are using the SAS-B scheme to prescribe for these conditions successfully, presumably based on their own research, patient demand, or trial and error (see Table 1). This includes conditions such as anxiety and post-traumatic stress disorder (PTSD) which together currently account for approximately 40 cannabis approvals per month; various movement disorders including Parkinson’s disease, as well as Alzheimer’s disease, Crohn’s disease and autism spectrum disorder.
What is the nature of the data being collected?
To prescribe via the SAS-B route, clinicians submit a standard application justifying the need for an unregistered medicinal cannabis product for an individual patient. The information provided to the TGA on all applications includes:
- The age and sex of the patient;
- The primary medical indication justifying the application;
- Details about their diagnosis and other co-morbid medical conditions, including the severity of their primary indication;
- Details of previously attempted and failed treatments, and justification as to why no other TGA-registered medicines are appropriate and
- Any prior SAS approval number (for renewals).
This information alone is quite informative and would provide a platform to better understand the types of patients receiving medicinal cannabis. Of particular interest would be if there are clear examples of registered drugs failing for certain types of conditions, such as opioid or gabapentinoid drugs for specific types of chronic pain.
Another key piece of information that could be extracted from the existing data is the number of renewals, or lack thereof, across indications. A renewal could be taken as a proxy for effectiveness of a medicinal cannabis intervention. The TGA has renewal information readily available as they request prior SAS approval numbers for each patient (if applicable).
Collectively, this information, if disseminated, could aid clinicians in making decisions about the likelihood of a medicinal cannabis intervention having an appreciable benefit in a particular condition. Similarly, if there is an absence of renewals, then this might indicate a low level of success, or highlight other issues with the medicinal cannabis framework such as the cost of these products to patients.
At the time of renewal, clinicians are obliged to provide some data to the TGA about the patient’s progress to rationalise the extension of their prescription. As per the SAS-B guidance document, “If an health practitioner submits a SAS application for the same product, patient and indication(s) – that is, a repeat application – they are expected to provide with that application the results of previous monitoring, including measures of patient response and safety parameters.” This implies that the TGA is already capturing some, albeit limited, data about patient experience with medicinal cannabis products for a range of conditions, including some where clinical evidence is lacking.
Some SAS-B data, such as that shown in Figures 1 and 2, are being made available to researchers (such as our team) but only through Freedom of Information (FOI) requests. But FOI requests are clearly a suboptimal way of acquiring and disseminating such information. Problems include: the cost and administrative burden to routinely request information; the delay in the supply of data (usually a minimum 1-2 month lag between request and receipt); the need for expertise to appropriately analyse the data in the technical format in which it is received; and the failure to address the public interest involved in having such data routinely updated and easily available from a well-maintained, easily-accessed, impartial platform. We believe it would be most suitable for the TGA, as a trusted and well-respected regulator, to publish the information and a summary analysis on its website for the use of health professionals and researchers.
Who is watching after medicinal cannabis prescription?
In the pharmaceutical company context with newly registered drugs there is mandatory post-market surveillance (or ‘pharmacovigilance’) of patients undertaken by the company supplying the drug. The unregistered nature of Australia’s medicinal cannabis products means this requirement is absent. So there is essentially no safety and efficacy follow-up data with the 50 or more medicinal cannabis products that patients are currently using.
Australia’s pharmacovigilance system relies entirely on drug companies to observe the performance and safety of their products once they are registered with the TGA. The TGA does not, and has no intention of initiating, its own pharmacovigilance program. The TGA’s stance on this issue is not surprising and is in line with their responsibilities as a regulator and not a drug sponsor.
In a typical situation, the pharmaceutical company selling the drug product is responsible, and legally obliged, to perform this data collection and analysis function. But medicinal cannabis is not a typical situation. Most medicinal cannabis companies supplying the Australian market appear unlikely to register their products with the TGA anytime soon.
Such registrations will likely be years from now which means that data arising from tens of thousands of approvals and renewals will go unexamined in the meantime. This is the point at which unusual and unique circumstances require exceptional duties to be performed; something we hope the TGA will recognise.
For example, approximately 30 patients per month are being prescribed medicinal cannabis products for anxiety and are presumably either discontinuing or renewing their prescription for various products. How many of these patients are finding these products efficacious for their symptoms? Which product has the greatest ‘success’ rate out of the options available? What are the most common side-effects and drug/drug interactions being observed? How many discontinue? What might be the ideal dose range for treating anxiety based on patient experience?
The TGA is acutely aware of the fragile state of evidence for medicinal cannabis in many conditions, having commissioned clinical guidance documents for several of these. In the TGA clinical guidance document on chronic non-cancer pain, they state that the evidence is still lacking for many pain conditions and as such,
“There is a need for larger trials of sufficient quality, size and duration to examine the safety and efficacy of medicinal cannabis use in [chronic non-cancer pain]”.
We are now in a situation where over 500 Australians are prescribed a medicinal cannabis product for pain every month (see Fig 2.) Systematic monitoring of these patients would assist in building this evidence base, even potentially informing future updates to the TGA’s guidance documents. In fact, the TGA have found analogous pharmacovigilance programs, in the form of registries, to be effective tools for assessing clinical safety in other therapeutic areas.
One example is joint replacement: the Australian Orthopaedic Association National Joint Replacement Registry provides comprehensive observational data which the TGA uses to routinely assess safety of prostheses on the market, informing clinical practice. A similar approach with medicinal cannabis products could provide the TGA the same opportunity to assess safety and response, in the absence of drug sponsors completing this.
The ‘Companion survey on the use of cannabis medicines in Germany’ is an example of such a national database. Launched in April 2017 this is a mandatory national non-interventional follow-up survey with a focus on collection and evaluation of anonymised treatment data using an online portal operated by the Federal Institute for Drugs and Medical Devices.
The survey has captured clinician-entered data from over 4,100 medicinal cannabis patients so far. This has been used to inform German physicians in prescribing decisions and in designing potential future trials, as well as guide insurance and government health bodies. A recently published interim evaluation of the database has provided useful insights into prescribing practices across different therapeutic areas, product information breakdowns, and also discontinuation rates due to lack of efficacy. Australian doctors could most certainly benefit from this type of resource.
One Australian State is attempting to fill the data gap
As of January 2019, researchers from the Office of Medicinal Cannabis in the Australian State of Victoria partnered with several Australian universities to initiate The Australian Medicinal Cannabis Safety and Effectiveness Study. Every patient prescribed Schedule 8 medicinal cannabis (which includes all medicinal cannabis products except for 98%-pure cannabidiol or ‘CBD’ products) in Victoria can enrol, alongside their prescribing doctor, to provide data about the patient’s concomitant medications, the condition they are treating, and how cannabis is affecting the progression of their disease and symptoms.
This State-driven approach faces its own problems. First, it requires doctors and patients to opt-in, while the national TGA systems are mandatory for doctors and their patients obtaining medicinal cannabis access. Second, the State is not notified by the TGA to provide authorisation for Schedule 4 cannabis medicines (containing 98% cannabidiol or ‘CBD’), so these approvals (which currently account for about one third of all approvals) are lost. Third, it is limited to doctors and patients in the single State of Victoria, thus missing out on the majority of Australian prescriptions.
Other State Health departments have been repeatedly approached to attempt the creation of a national data collection initiative. However, to date, no other States have signed up and several have displayed active disinterest in study participation. This apparent bureaucratic indifference is concerning but could be bypassed if the TGA were to implement a national system that builds upon the existing framework they have in place.
Aside from this single State project there are a number of observational cohort studies emerging from the medicinal cannabis access clinics that have recently been established in various Australian cities. Such studies are either being sponsored by the clinic or the individual cannabis companies supplying the products. This is helpful market research, yet potentially distorted by commercial conflicts of interest around collection. Furthermore, these organisations are not required to publish or to make the source data publicly available for use in clinical guidance, and therefore cannot be relied upon as a consistent source of data.
A missed opportunity
The longer the TGA waits to implement a suitable data capture system for medicinal cannabis prescriptions in Australia, the more potentially informative data will be lost. The time to implement such a system is now. More than half of the total number of medicinal cannabis SAS-B approvals over the last 30 months have been issued in 2019. Monthly approval rates are escalating so that by the end of 2019 we predict several thousand approvals per month for new and returning patients. Putting in place a systematic way to collect, analyse and disseminate data from these patients ahead of this potential flood of approvals is key.
We reached out to the TGA for comment and they clarified their position; “Reflecting the TGA’s decision under the Therapeutic Goods Act 1989, whether to give SAS-B approval to a medical practitioner for medicinal cannabis products for a particular patient, the TGA asks the practitioner to confirm that the benefits outweigh the risks for that patient. It does not collect historical observations of the efficacy of that product. It is also not authorised to require an applicant medical practitioner to report to it the efficacy, in the patient for whom the approval is sought, of the approved use.
Subject to the Therapeutic Goods Act, the TGA may not separately use that information for an altogether different purpose. This ‘SAS-B data’ is also separate to data collected under the adverse event reporting regime. The TGA is also not authorised to carry out clinical trials or prepare risk management plans.”
To put such a data capture system in place would admittedly not be free and this is made even more difficult by the fact that the TGA is mandated to operate on a cost-recovery basis. Although much useful information is already being captured by the TGA, to extend this into a more rigorous data capture process, such as a registry, would require additional resources and legislative amendments to the Therapeutic Goods Act.
Australian governments do however seem willing to fund other medicinal cannabis-related projects and amend legislation where necessary. The TGA created and implemented an online portal to streamline SAS-B requests in mid-2018 which has improved access significantly, likely at considerable expense. Furthermore various State Health Departments have directly funded several medicinal cannabis clinical trials, not to mention the significant AUD $6 million (~€3.6m) investment by the New South Wales department of Health to establish a call centre to answer people’s questions about medicinal cannabis.
With the resources and infrastructure Australia has at its disposal, there is no reason that a highly informative system of data capture around medicinal cannabis patients cannot be achieved at a national level.
References
- Therapeutic Goods Administration (TGA). (2019). Access to medicinal cannabis products. [online] Available at: https://www.tga.gov.au/access-medicinal-cannabis-products-1
- Lintzeris, N., Driels, J., Elias, N., Arnold, J., McGregor, I. and Allsop, D. (2018). Medicinal cannabis in Australia, 2016: the Cannabis as Medicine Survey (CAMS-16). Medical Journal of Australia, 209(5), pp.211-216
- Karanges, E., Suraev, A., Elias, N., Manocha, R. and McGregor, I. (2018). Knowledge and attitudes of Australian general practitioners towards medicinal cannabis: a cross-sectional survey. BMJ Open, 8(7)
- The University of Sydney. (2019). How to get medicinal cannabis. [online] Available at: https://sydney.edu.au/lambert/how-to-get-medicinal-cannabis.html
- Therapeutic Goods Administration (TGA). (2019). Authorised Prescribers. [online] Available at: https://www.tga.gov.au/form/authorised-prescribers
- Therapeutic Goods Administration (TGA). (2019). Special Access Scheme. [online] Available at: https://www.tga.gov.au/form/special-access-scheme
- FreshLeaf Analytics (2019). Australian Medicinal Cannabis Market Patient, Product and Pricing Analysis. Q1 2019
- Therapeutic Goods Administration (TGA). (2019). Medicinal cannabis – guidance documents. [online] Available at: https://www.tga.gov.au/medicinal-cannabis-guidance-documents
- Therapeutic Goods Administration (2019). Freedom Of Information request 1081-1819.
- Special Access Scheme – Category B. (2019). [online] Available at: https://www.tga.gov.au/sites/default/files/special-access-scheme-category-b.pdf
- Therapeutic Goods Administration (TGA). (2019). Information for health practitioners. [online] Available at: https://www.tga.gov.au/book-page/information-health-practitioners
- Therapeutic Goods Administration (TGA). (2019). Guidance for the use of medicinal cannabis in the treatment of chronic non-cancer pain in Australia. [online] Available at: https://www.tga.gov.au/publication/guidance-use-medicinal-cannabis-treatment-chronic-non-cancer-pain-australia
- Australian Orthopaedic Association National Joint Replacement Registry. (2019). Home. [online] Available at: https://aoanjrr.sahmri.com/
- Schmidt-Wolf, G. and Cremer-Schaeffer, P. (2019). Begleiterhebung zur Anwendung von Cannabisarzneimitteln in Deutschland – Zwischenauswertung. Bundesgesundheitsblatt – Gesundheitsforschung – Gesundheitsschutz.
- NCPHN. (2019). Australian Medicinal Cannabis Safety and Effectiveness Study – NCPHN. [online] Available at: https://ncphn.org.au/archives/news/australian-medicinal-cannabis-safety-and-effectiveness-study-xhp-x7
- Therapeutic Goods Administration (TGA). (2019). Special Access Scheme · My SAS hub. [online] Available at: https://sas.tga.gov.au/
- Centre for Medicinal Cannabis Research and Innovation NSW (2019). $6 Million Cannabis Medicines Hotline Opens. [online] Available at: https://www.medicinalcannabis.nsw.gov.au/home/featured-news/NSW-CMAS
| Approved indication | Number of SAS-B approvals |
| Cancer pain | 439 |
| Anxiety | 123 |
| Post-traumatic Stress disorder (PTSD) | 71 |
| Sleep disorders including insomnia | 22 |
| Parkinson’s Disease | 18 |
| Mood Disorder – Unspecified | 17 |
| Migraine | 17 |
| Alzheimer’s Disease | 13 |
| Tremor | 12 |
| Dystonia | 11 |
| Tourette’s Syndrome | 10 |
| Behavioural problems (including aggression & agitation) | 10 |
| Rare neuromuscular disorders (Issacs, Kearns-Sayre, Motor Neuron, Stiff persons, Progressive Supranuclear Palsy) | 10 |
| Huntington’s Chorea | 9 |
| Movement disorder – Unspecified | 8 |
| Gastrointestinal Disorders (Ulcerative colitis, Crohn’s disease, Irritable bowel syndrome) | 7 |
| Autism Spectrum Disorder | 7 |
| Restless Legs Syndrome | 3 |
| Panic disorder – Unspecified | 2 |
| Dyskinesia | 2 |
| Anaemia | 1 |
| Blood glucose management | 1 |
| Dyspnea | 1 |
| Depression | 1 |
| Oscillopsia | 1 |
| Myoclonic disorder | 1 |
| Neurological disorder – Unspecified | 1 |
| Muscular Dystrophy | 1 |
Dr Melissa Benson
Clinical Research Fellow
Rhys Cohen
Senior Project Officer
Lambert Initiative for Cannabinoid Therapeutics
Brain and Mind Centre
The University of Sydney
lambert.initiative@sydney.edu.au
Tweet @Lambert_Usyd
https://sydney.edu.au/lambert
Please note, this article will appear in issue 10 of Health Europa Quarterly, which is available to read now.









