Defence Therapeutics Inc., a Canadian biopharmaceutical company developing novel immune-oncology vaccines and drug delivery technologies, is pleased to announce that its second-generation ARM-X anti-cancer vaccine is therapeutically effective against pre-established ovarian cancer (ID8 model) when combined with the anti-PD-1 immune-checkpoint inhibitor.
Using Defence’s Accum® platform, Defence previously demonstrated that AccuTOX® treatment of MSCs results in the induction of antigen cross-presentation capacity (ARM-X cells), which can mount potent anti-tumoral responses in animal pre-clinical models. This was previously achieved using various cancer models including solid T-cell lymphoma, melanoma and pancreatic cancer. Defence just completed an additional study where animals with pre-established ovarian cancer responded to a combination therapy including ARM-X and anti-PD-1. The latter group prolonged animal survival beyond 80 days post-vaccination, and it led to a complete response in almost all treated animals as shown in Figure 1.
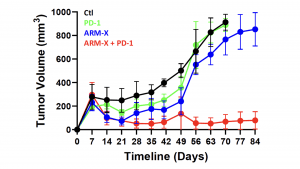
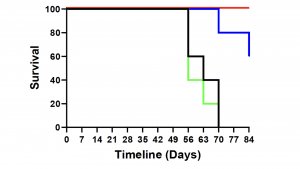
“This is the 4th cancer model that we efficiently targeted using our ARM-X antic-cancer vaccine. The purpose of testing our vaccine in various models is to highlight how ARM-X can be adapted to the needs of any patient, no matter the type of cancer, given that we have access to a tumour biopsy,” commented Sebastien Plouffe, Chief Executive Officer of Defence Therapeutics.
One of the major advantages of Defence’s ARM-X vaccine is the need for lower antigen amounts to manufacture the vaccine. This is important as it avoids the need for a big tumour sample in the vaccine generation. Defence is currently testing its ARM-X vaccine on the colon as an additional indication. These results will set the target indication for the Phase I-IIa trials and they also show how versatile and adaptable the ARM-X anti-cancer vaccine be.