
Leading biotechnology company Defence Therapeutics explores its growing patent portfolio for the company’s flagship AccumTM-based platform.
Defence Therapeutics is harnessing the power of AccumTM to design revolutionary therapeutics and prophylactics against cancers and infectious diseases. In 2016, a groundbreaking investigation identified AccumTM’s ability to enhance the potency of antibody-drug conjugates (ADCs) greatly.
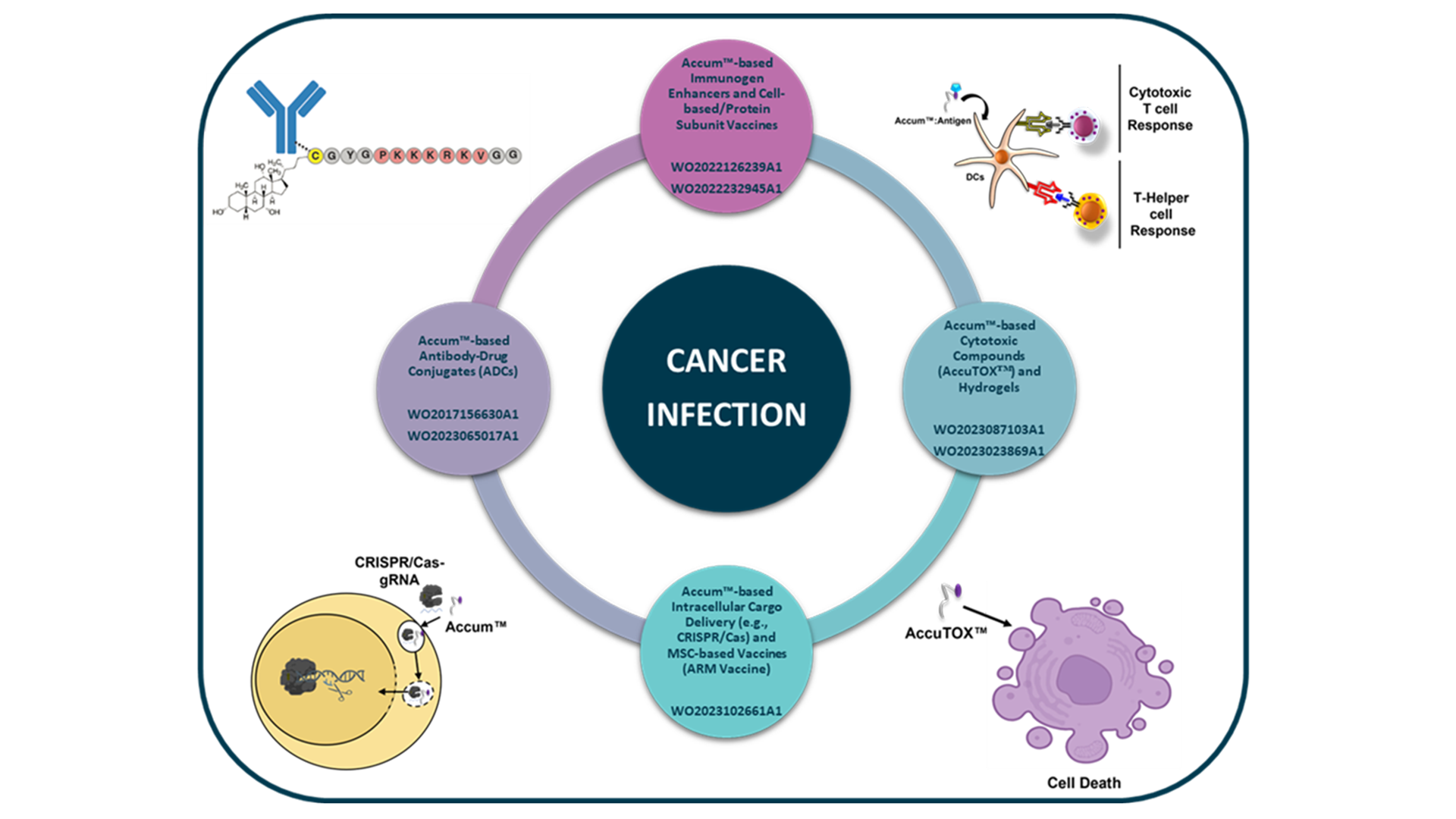
Defence’s continued research and development efforts have given rise to a robust and versatile platform, including novel applications and second-generation molecular entities having greater potency. Defence’s aggressive pursuit of patent protection is in lockstep with each innovation. Seven distinct patent families relating to four different central functions of Accum™ have been published to date.
Patents for ADCs
Defence’s ADC platform technology is based on the ability of the original Accum™ to form potent antibody-drug conjugates, leading to targeted cellular accumulation of a drug was demonstrated in international (PCT) patent application WO/2017/156630A1, with patents granted in the United States (US 11,352,437), Japan (JP 7,126,956), and Israel (IL 261765), and patent applications currently pending in Europe, Canada, and Australia.
Second-generation AccumTM-based ADCs are being pursued in the recently filed PCT application WO/2023/065017A1, currently in the international phase. In these patent families, Accum™ molecules were covalently linked to antibodies or ADCs (e.g., trastuzumab-DM1 ADC). They were shown to enhance their delivery and cytotoxicity in various cancer cell lines compared to controls.
Enhancing vaccine design
Defence’s vaccine platform technology is based on the discovery that Accum™ and variants thereof have the ability to enhance the presentation of various tumour, viral, or bacterial antigens by anti-gen-presenting cells, either by covalent conjugation to or simple admixture with the antigen.
PCT patent applications WO/2022/126239 A1 and WO/2022/232945 A1 were filed covering this technology, with respective US patents (US 11,291,717 and US 11,612,651) being already granted and corresponding patent applications currently pending worldwide.
In these patent families, the Accum™ technology was shown to produce very powerful therapeutic and prophylactic protein subunit- and cell-based vaccines for treating or preventing infectious diseases, including SARS-CoV-2 and cancers such as lymphoma.
Patents for the AccuTOX™ platform
Defence’s AccuTOX™ platform technology is based on the ability of some Accum™ variants, either as standalone agents or in combination with other cytotoxic agents, to induce cancer cell death by triggering heightened metabolic stress and the production of reactive oxygen species, as shown in PCT patent application WO/2023/087103 A1.
In this patent family, the AccuTOX™ technology induced cell death in a plurality of different tumour cell lines in vitro, in many cases via apoptosis. It was shown to reduce lymphoma tumour volumes and enhance survival in treated mice compared to controls.
In a separate discovery, standalone Accum™-based agents were found to form hydrogels (WO/2023/023869 A1) upon dissolution and subsequent incubation in different aqueous solvents, providing further avenues in the innovation of drug delivery systems.
Additional patents
Finally, the ability of Accum™ and its variants to efficiently deliver multiple therapeutically relevant cargoes, including polynucleotides, recombinant proteins, and even nucleoprotein complexes, into the cytosol or nucleus of cells is demonstrated in PCT patent application WO/2023/102661 A1.
Importantly, in this patent family, the Accum™ technology was shown to successfully deliver functional CRISPR/Cas9 and guide RNA ribonucleoprotein complexes into the nucleus of cells, leading to intriguing commercial avenues in the field of genome editing.
Furthermore, this patent family further contains in vivo data demonstrating the efficacy of Defence’s AccumTM-based technology in transforming traditionally non-antigen presenting and immune quiescing mesenchymal stem cells (MSCs) into efficient antigen presenters and immunostimulatory cells, in the form of a highly efficacious anti-cancer cellular vaccine.
These MSC-based cellular vaccines led to Defence’s potent second-generation MSC-based therapeutic ARM vaccine that is on its way into Phase I clinical trials.
Sebastien Plouffe, President and Chief Executive Officer of Defence, commented: “Defence’s proactive and aggressive approach in securing patent protection for all aspects of our technologies serves to maximise the commercialisation potential and increase the company’s intrinsic value as we continue to march toward the goal of bringing our innovative therapeutic technologies to market and making a lasting global impact on the lives of patients.”
Defence patent families are managed by ROBIC LLP, a Montreal-based law firm specialising in all facets of intellectual property in many different fields since 1892, notably in biotechnology and pharmaceuticals.









