
Defence Therapeutics Inc. is pleased to announce that it successfully submitted an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) for its ACCUM-002TM Dimer CDCA-SV40 commonly named ‘AccuTOX®’, an injectable anticancer molecule, for the treatment of solid cancer tumours.
AccuTOX® is a derivative of the initial Accum® molecule, which has been reported to target cancer on multiple fronts. AccuTOX® disrupts endosomal membranes, resulting in impaired intracellular transport mechanisms.
AccuTOX® also triggers genotoxic effects, blocks DNA repair mechanisms generally used by cancer cells to repair its damaged genome and induces a form of immunogenic cell death capable of turning ‘ON’ the immune system.
When previously tested in preclinical animal models under the supervision of Dr Moutih Rafei, AccuTOX® impaired tumour growth, resulting in 70-100% survival of animals with solid T-cell lymphoma, melanoma, or breast cancer.
Proving AccuTOX® can become a commercial tumour treatment
The IND application includes data, reports, and overview summaries of numerous studies to evaluate the pharmacology, pharmacokinetics, and toxicology of AccuTOX® in vitro and in vivo, including cancer models.
In addition, the application describes the manufacture of the drug substance and drug product used in human clinical trials.
The primary purpose of the IND is to share with the FDA the extensive non-clinical data supporting an acceptable safety profile when AccuTOX® is first administered to humans. The FDA will review the application and determine the acceptability of the data before Defence begins the Phase I clinical trial, which could be as early as Q1-Q2 2024.
“We are thrilled and excited that Defence has successfully submitted its first IND, which represents an important milestone towards advancing AccuTOX® into the clinic. We look forward to working with clinical investigators at the City of Hope to study this important and novel candidate for treating melanoma and potentially other solid tumours,” said Sébastien Plouffe, President & CEO of Defence Therapeutics.
“With the continued difficulties encountered in the oncology clinic, we believe that the therapeutic use of AccuTOX® provides a novel and powerful approach to combat cancer,” he added.
Upcoming Phase I clinical trial
The primary objective of this upcoming Phase I clinical trial, when approved, is to identify the best therapeutic dosing range that would allow clinicians to co-administer the AccuTOX® compound with Opdulag®, a BMS product containing both anti-LAG3 and anti-PD-1.
Several secondary parameters, including therapeutic efficacy, will be monitored in treated patients in preparation for a Phase IIa trial on a basket of tumours.
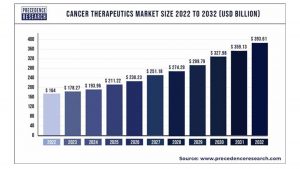
According to Precedence Research, the global cancer therapeutics market size is expected to be worth around $393.61bn by 2032 from $164bn in 2022, growing at a CAGR of 9.20% during the forecast period from 2023 to 2032.










