FebriDx® rapid point of care triage testing can help prevent the spread of infection and reduce risk in clinical settings.
The coronavirus 2019 (COVID-19) pandemic is placing a significant strain on all healthcare settings. Reverse transcriptase polymerase chain reaction (RT-PCR) testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is available but has a long turnaround time (hours to days) (Public Health England 2020; Devine 2020). This delay in diagnosis slows patient isolation decisions and thereby places patients at risk for cross infection.
Molecular SARS-CoV-2 tests are also known to have suboptimal performance, with reported sensitivities ranging from 62% to 80% during the first seven days of symptom onset (Kucirka et al. 2020), therefore requiring repeat testing, which further places patients at risk of cross-infection.
Rapid antigen tests are available for SARS-CoV-2; however, whilst they are rapid and easy to use, their performance in a real world setting has been shown to be only 23% to 30% sensitive (Scohy et al. 2020; Blairon et al. 2020) and therefore negative results must be backed up by RT-PCR testing.
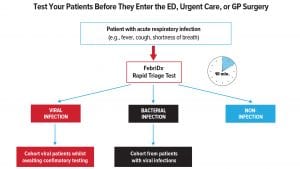
The COVID-19 pandemic has resulted in a significant influx of patients which has overwhelmed healthcare systems, underscoring the need to rapidly triage patients at the point of entry. Point of care (POC) tests that rapidly differentiate bacterial from viral infections would be useful to cohort potential COVID-19 patients away from negative patients whilst preventing the delay in management and treatment for bacterial infections. Alternative rapid accurate diagnostic tests are urgently needed by healthcare professionals.
UK hospital studies
Two UK hospital studies prospectively evaluated the utility of FebriDx® to rapidly identify viral cases requiring immediate isolation and confirmatory molecular testing from non-infectious patients or bacterial infections requiring antibiotics.
Utility of FebriDx in early identification of possible COVID-19 infection: Karim et al.
The long turnaround time and false negative results of RT-PCR currently result in delays in diagnosis and patient isolation decisions which place patients at risk of cross-infection. Karim et al. evaluated the utility of FebriDx on 48 patients suspected to have a COVID-19 infection to reduce time to presumed diagnosis and allow for appropriate isolation in suspected COVID-19 cases. Using the European Centre for Disease Prevention and Control (ECDC) COVID-19 case definitions, 35 patients were confirmed to have a final diagnosis of COVID-19.
FebriDx identified 35/35 (100%) of COVID-19 infections, whereas initial PCR SARS-CoV-2 test only identified 29/35 (82.9%). FebriDx provided results in 10 minutes whilst the SARS-CoV-2 RT-PCR had a 48-hour turnaround time. The use of FebriDx as an initial triage test may have prevented COVID-19 negative patients being exposed to COVID-19 positive patients caused by the delay in molecular test results. Single biomarker C-reactive protein (CRP), Procalcitonin or leukocyte counts were unable to differentiate viral from bacterial infections due to the considerable overlap in values.
During viral pandemics it is also imperative to ensure that patients with bacterial infections are not missed. In the study, 8/48 of the patients (negative for COVID-19) were shown to have a bacterial infection. FebriDx detected 8/8 bacterial infections (100% sensitive; 92.5% specific).
Karim et al concluded that ‘FebriDx can be successfully deployed as a reliable triage test amongst hospital or ED patients suspected to have COVID-19’.
Diagnostic accuracy of the FebriDx host response point-of-care test in patients hospitalised with suspected COVID-19: Clark et al.
The management of the COVID-19 pandemic is hampered by the long delays associated with centralised laboratory resulting in poor patient flow and spread of infection therefore alternative rapid accurate diagnostic tests are urgently required. Clark et al. prospectively evaluated the real world diagnostic accuracy of the FebriDx test for the identification of COVID-19 in patients hospitalised with COVID-19 during the first wave of the pandemic.
FebriDx was shown to be 93% (110/118) sensitive and 86% (112/130) specific for identification of COVID-19 infections compared to PCR. FebriDx results were available within 10 minutes compared to 23.4 hours (17.2 to 31.1) for laboratory PCR testing. Several patients with FebriDx viral positive results (negative by PCR) had classical radiological features of COVID-19, thus were likely to be true positives despite negative PCR results. By comparing FebriDx to the ECDC COVID-19 case definition, FebriDx would have been 93.6% (117/125) sensitive and 91.1% (112/123) specific, with PCR being 94.4% sensitive (118/125).
Due to the high sensitivity and negative predictive value (NPV), FebriDx viral negative patients can be rapidly cohorted in non-COVID-19 areas allowing FebriDx viral positive patients to be immediately isolated whilst awaiting confirmatory testing from a PCR or CT scan.
FebriDx NPV was calculated to be 99% both at 20% and 10% COVID-19 prevalence, leading to the conclusion that a negative FebriDx will be a useful rule-out test.
The FebriDx test was shown to be highly accurate in rapid identification of COVID-19 infections and could be rapidly deployed as a front door triage tool in hospitals and urgent care centres to overcome current issues of delayed diagnosis from PCR testing. FebriDx as a rapid triage tool that detects both influenza and COVID-19 is likely to be of utility in the coming winter months.
FebriDx: the front door triage test
FebriDx allows clinicians to:
- Rapidly identify viral patients;
- Facilitate immediate patient isolation;
- Ensure patients with bacterial infections are not missed; and
- Rule out non-clinically significant infection.
Save time and money with FebriDx triage
Rapid triaging with FebriDx can prevent the spread of infection. Direct medical costs for a single COVID-19 infection are estimated at $3,045 (Bartsch et al 2020) FebriDx can prevent COVID-19 negative patients being exposed to COVID-19 positive patients caused by the delay in PCR testing results. FebriDx can also assist the triage of patients which require confirmatory molecular testing.
About FebriDx
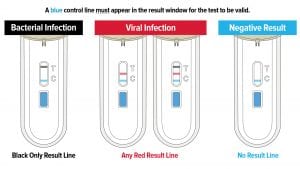
FebriDx is the first and only rapid POC test to differentiate a viral from bacterial acute respiratory infection (ARI) through the simultaneous detection of both Myxovirus resistance protein A (MxA) and C-reactive protein (CRP) directly from a fingerstick blood sample in just 10 minutes. FebriDx is an ‘all in one’ style portable POC test which incorporates an onboard fingerstick sample collection.
MxA is an intracellular protein that becomes elevated only in the presence of acute viral infection (Engelmann et al. 2015, Nakabayashi et al. 2006); and CRP is an acute phase inflammatory protein that is elevated in the presence of a systemic bacterial and/or viral infection (Falk and Fahey 2009). FebriDx, with its dual biomarker technology, optimises the sensitivity and specificity of both markers to accurately and reliably differentiate viral and bacterial ARIs (Shapiro et al. 2018, Self et al. 2017).

Proven performance
Clinical performance from a prospective multicentre US clinical trial concluded FebriDx to be 95% sensitive, 94% specific and have a negative predictive value of 99% to exclude a bacterial infection and a positive predictive value (PPV) of 91% to confirm viral infection (Shapiro et al. 2018; Self et al. 2017). In a UK outcome study in a General Practice (GP) setting, FebriDx improved the clinical management decision in 48% of patients tested including two patients who were clinically presumed viral, but FebriDx determined a bacterial infection. One of these patients was admitted to the hospital with sepsis. FebriDx also reduced unnecessary antibiotic prescriptions by 80% without any adverse results (Davidson 2017).
FebriDx technology
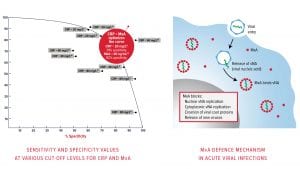
FebriDx utilises dual biomarker technology to deliver high sensitivity and specificity for both viral and bacterial ARI. A single biomarker approach is not sufficiently accurate to differentiate viral from bacterial infection due to an overlap in values. With single biomarkers it is possible to only select a cut-off where either the sensitivity or specificity is high, but not both, which could lead to false negative or false positive results.
FebriDx simultaneously detects elevated levels of MxA and CRP from fingerstick blood to accurately and reliably differentiate viral and bacterial ARI. By combining CRP, an acute phase protein, and MxA, an intracellular blood protein which is only elevated in acute viral infections, FebriDx optimises the sensitivity and specificity of both biomarkers.
CRP is an acute phase inflammatory protein that is elevated in both viral and bacterial infections. While bacterial infection is a potent stimulus of marked CRP elevation, CRP levels may increase over 100 mg/L with adenovirus, influenza and now COVID-19 infections (Joseph and Godofsky 2018; Clark et al. 2020). MxA is an intracellular blood protein that is stimulated by interferon (IFN) alpha/beta cells. IFN cells are induced by viruses and form an essential part of the immune system’s defence against viral infections.
MxA protein becomes elevated only in the presence of acute viral infections and not in bacterial infection. MxA has a fast induction time of one to two hours and a long half-life of 2.3 days, making it an ideal biomarker for acute viral infections (Nakabayashi et al. 2006).
FebriDx dual biomarker technology utilises CRP at a 20 mg/L cut-off and confirms the specificity with the MxA biomarker at a 40 ng/mL cut-off to deliver both sensitivity and specificity in detecting and differentiating viral and bacterial ARIs.
Antimicrobial stewardship and the pandemic
ARIs – which include pharyngitis, rhinosinusitis, acute cough, bronchitis, influenza and pneumonia – are the most common reason patients seek healthcare worldwide. Most ARIs are of viral origin; however, the signs and symptoms of both viral and bacterial infections are similar. The resulting diagnostic uncertainty and patient pressure to receive antibiotics leads to the over prescription of antibiotics. It is estimated that over 50% of antibiotics prescribed for ARIs are unnecessary and this is the leading cause for antimicrobial resistance (AMR). Currently 700,000 people die from drug-resistant bacteria each year, with deaths from AMR predicted to increase to 10 million deaths by 2050 unless countermeasures are taken (O’Neill 2018).
The COVID-19 pandemic reinforces the need for effective antimicrobial stewardship and the importance to be able to rapidly differentiate viral from bacterial ARIs. The World Health Organization (WHO) has issued guidance on the management and treatment of patients with COVID-19, advocating specific measures to address antibiotic use during the pandemic. WHO published that 72% (1450/2010) of hospitalised COVID-19 patients had received antibiotics, yet only 8% (62/806) were shown to have a bacterial or fungal co-infection (Getahun et al. 2020).
Don’t miss a bacterial infection
During the pandemic it is critical that patients with bacterial infections, including bacterial community-acquired pneumonia (CAP), are not missed. The signs and symptoms of COVID-19 are very similar to bacterial CAP; and the focus on testing for SARS-CoV-2 can cause a bacterial infection to be overlooked or diagnosis delayed, which could mean delayed antibiotic treatment. Bacterial CAP is the only ARI for which a delayed onset of antibiotics is associated with increased mortality.
Conclusion
The long turnaround times of RT-PCR SARS-CoV-2 tests cause delays in identifying COVID-19 positive and negative patients which leads to the subsequent potential for spread of infection. The situation is further compounded by the limited availability of single isolation units, meaning that patients are temporarily held in assessment areas or inpatient wards until results are available.
The ability to triage patients at the first point of contact, when clinical management and therapeutic decisions are being made regarding the need for isolation, confirmatory testing, and treatment of COVID-19 and bacterial infections, is a critical component to our national pandemic response. There is an urgent need to implement the proposed strategy to combat the current outbreak. FebriDx is the first and only rapid test to differentiate a viral from bacterial acute respiratory infection. FebriDx can enable effective triaging, so viral negative patients can be rapidly cohorted in non-COVID-19 areas allowing viral positive patients to be immediately isolated while awaiting confirmatory PCR testing (with an average turnaround of 48 hours), in order to prevent the spread of infection.
FebriDx has been demonstrated to be highly accurate at rapidly detecting COVID-19 infections, including identifying infections which have been missed by RT-PCR testing; and can be rapidly deployed as a front door triage tool in hospitals and urgent care centres to overcome current issues of delayed diagnosis from PCR testing. FebriDx will be similarly important outside of the hospital in community COVID-19 ‘hot hubs’, care homes and GP practices.
References
1 Public Health England (PHE). Coronavirus (COVID-19): getting tested. April 15, 2020. Accessed on July 14, 2020.
2 Devine, C., Covid-19 testing: A spike in demand, a delay on results, in CNN. July 7, 2020. Accessed on July 22, 2020.
3 Kucirka, L. M. et al. 2020. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction–Based SARS-CoV-2 Tests by Time Since Exposure, Annals of Internal Medicine.
4 Scohy, A. et al. 2020. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis, Journal of Clinical Virology: 104455.
5 Blairon, L. et al.2020. Implementation of rapid SARS-CoV-2 antigenic testing in a laboratory without access to molecular methods: experiences of a general hospital, Journal of Clinical Virology: 104472.
6 Karim, N. et al. 2020. Utility of FebriDx® in early identification of possible COVID19 infection.
7 The European Centers for Disease Prevention and Control (ECDC). Case definition for coronavirus disease 2019 (COVID-19), as of 29 May 2020.
8 Clark, T. W. et al. 2020. Diagnostic accuracy of the FebriDx® host response point-of-care test in patients hospitalised with suspected COVID-19, Journal of Infection.
9 Bartsch S. M. et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff. 2020;39(6):927–935.
10 Engelmann, I. et al. (2015). Diagnosis of viral infections using myxovirus resistance protein A (MxA). Pediatrics, 135(4), e985-e993.
13 Falk, G. and Fahey, T. 2009. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies, Family practice, 26: 10-21.
14 Shapiro, N. et al. 2018. A prospective, multi-centre US clinical trial to determine accuracy of FebriDx® point-of-care testing for acute upper respiratory infections with and without a confirmed fever, Annals of Medicine, 50: 420-29.
15 Self, W. et al. 2017. Diagnostic accuracy of FebriDx®: a rapid test to detect immune responses to viral and bacterial upper respiratory infections, Journal of clinical medicine, 6: 94.
16 Davidson, M. 2017. FebriDx® Point-of-Care Testing to Guide Antibiotic Therapy for Acute Respiratory Tract Infection in UK Primary Care: A Retrospective Outcome Analysis. J Infect Dis Preve Med, 5: 165.
17 Joseph, P. and Godofsky, E. 2018. Outpatient antibiotic stewardship: a growing frontier—combining myxovirus resistance protein a with other biomarkers to improve antibiotic use. In Open forum infectious diseases, ofy024. Oxford University Press US.
18 Nakabayashi, M. et al. 2006. MxA-based recognition of viral illness in febrile children by a whole blood assay. Pediatric research, 60: 770-74.
19 O’Neill, Jim. 2018. Tackling drug-resistant infections globally: Final report and recommendations. 2016, HM Government and Wellcome Trust: UK.
20 Getahun, H. et al. Tackling antimicrobial resistance in the COVID-19 pandemic. Bulletin of the World Health Organization 2020;98:442-442A.
John Wisson
Lumos Diagnostics
john.wisson@lumosdiagnostics.com
www.FebriDx.com
This article is from issue 14 of Health Europa. Click here to get your free subscription today.