The Safer Pharma campaign to eliminate pharmaceuticals in the environment
Adela Maghear outlines the dangers of pharmaceuticals in the environment and the measures needed to reduce their threat to human and environmental health.
Pharmaceutical pollution...
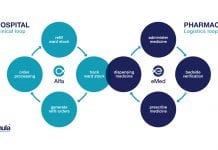
Thula – Nordic Source Solutions
Thula-Nordic Source Solutions explains how it is taking charge of medicine management through innovative technologies.
Thula-Nordic Source Solutions is a dynamic software product development and consulting...
The developing role of hospital pharmacists
Joan Peppard, the immediate past president of the European Association of Hospital Pharmacists, discusses the evolution of the hospital pharmacy specialist.
The prescribing of medication...
Strong rise in number of children given antidepressant prescriptions
The number of children in England, Scotland and Northern Ireland being give antidepressant prescriptions has seen a substantial increase over the last three years,...
The Precision Medicine and Biomarkers Leaders Summit 2018
Leading pharmaceutical and biotech companies worldwide are gathering for the Precision Medicine and Biomarkers Leaders Summit 2018. We provide an overview of the event.
In...
The past, present and future of Wageningen Bioveterinary Research
Dr Riks Maas and Professor Jeroen Kortekaas discuss the pioneering work of Wageningen Bioveterinary Research in the fields of viral vaccine development and animal...
World Vaccine Congress Europe 2018
The World Vaccine Congress Europe is the place to be for the latest news and developments in vaccine research – from antimicrobial resistance to...
The pharmaceutical industry responds to the Brexit White Paper
Mike Thompson, chief executive of the Association of the British Pharmaceutical Industry (APBI) and BioIndustry Association’s (BIA) chief executive Steve Bates have issued a...
EMA: Ramp up efforts to ensure EU medicine supply post-Brexit
The European Medicines Agency (EMA) has warned that some companies need to take further action to safeguard the EU medicine supply once the UK...
Medicines for Europe: better access, better health
Medicines for Europe president Marc-Alexander Mahl outlines the challenges facing the generic, biosimilar and value-added medicines industry and its role in achieving sustainable healthcare.
Medicines...
Antibiotic treatment should stop to avoid resistance “tipping point”
New research from the University of Exeter, UK, shows that if antibiotic treatment continues to be used, patients will pass the “tipping point” of...
Health outcomes and the community pharmacist
Ilaria Passarani, secretary general of PGEU, reflects on the invaluable role of the community pharmacist in improving patient health outcomes in Europe.
Every day, some...
Welcome to the Kuopio Center for Gene and Cell Therapy
The Kuopio Center for Gene and Cell Therapy is home to the research and development of new innovative medicines.
The Kuopio Center for Gene and...
Innovative tissue analysis: Munich spin-off project receives Helmholtz funding
Tissue analysis is an essential tool for investigating diseases and is a mainstay in the field of diagnostics. Conventionally, tissue is fixed, sliced into...
AMPK activator O304 – a novel treatment for Type 2 diabetes and diabetic kidney...
The high and increasing unmet patient need in diabetic kidney disease demands new drugs. Betagenon AB has the answer.
Globally, approximately 40% of Type 2...
European Medicines Agency prepares pharmaceutical companies for Brexit
The European Medicines Agency (EMA) and the European Commission have updated their guidance which will help pharmaceutical companies prepare for the UK’s withdrawal from...
Antibody therapy for migraine prevention set for marketing authorisation
The first monoclonal antibody therapy for migraine prevention, Aimovig, has been recommended for marketing authorisation by the European Medicines Agency's (EMA) Committee for Medicinal...
Oesophageal cancer can be prevented by taking two medications
Taking a combination of an anti-reflux medication and a low dose of aspirin can prevent oesophageal cancer in people at higher risk, according to...
Can antidepressants increase the risk of weight gain?
A new study published in The BMJ today has found that long term use of antidepressants is linked to the increase in risk of...
European Commission approves single pill for HIV-1 treatment
The Janssen Pharmaceutical Companies of Johnson & Johnson have today announced that the European Commission has approved JULUCA® (dolutegravir/rilpivirine), a once daily single pill...