The group of Dr Maija Hollmén at Turku University, Finland, attempts to make cold tumours hot by targeting the tumour-supporting functions of innate immune cells, thus enhancing cancer immunotherapy.
Several lines of evidence have led to the general acceptance that cancer and inflammation are connected, which could play a key role in improving cancer immunotherapy. For example, chronic inflammatory diseases have been known to increase the incidence of cancer and the use of non-steroidal anti-inflammatory drugs reduce the risk of developing cancer as well as decreasing the mortality caused by certain cancers. On the other hand, tumours that have no underlying inflammatory conditions, genetic events such as oncogene mutations, and chromosome rearrangements increase the production of inflammatory mediators that build up an inflammatory tumour microenvironment for cancer to progress.1
Regardless of the initial tumour-promoting event, a common feature of cancer-related inflammation is the presence of cells of the mononuclear phagocytic lineage that differentiate into specialised subpopulations of macrophages with diverse effects on malignant progression.
Balance is everything
The progression of cancer depends on the quality and quantity of anti-tumour immune responses. Lymphocytes continuously patrol through the blood, lymphoid tissues, and lymphatic vasculature during cancer immune surveillance. Pro-inflammatory and cytotoxic immune responses are essential in limiting tumour progression, whereas anti-inflammatory immune cell types often paradoxically promote tumour growth. For instance, regulatory T-cells (Treg) and alternatively activated macrophages can cause immunosuppression, which is one of the main obstacles in successful cancer treatment. Thus, the development of strategies targeting immunosuppressive cells, molecules, and pathways with less toxicity and with a more universal role in tumour progression is an unmet clinical need. Possibilities to control the presence of suppressive cells within tumours are key factors in opening new avenues to fight against cancer growth.
Macrophages form the first line of defence towards invading pathogens and they have a crucial role in maintaining tissue homeostasis. Macrophages have been called the Swiss Army knife of the immune system since they have a large repertoire of functions in immune activation and resolving inflammation. Macrophages can promote immunity against neoplastic cells by inducing T-cell recruitment and activation. However, their presence in the tumour microenvironment has been associated with poor prognosis in several tumour types. In fact, tumour-associated macrophages (TAMs) have been shown to promote cancer cell growth and dissemination as well as angiogenesis and immunosuppression. In addition, TAMs have been shown to alter responses to hormones and chemotherapeutic agents.
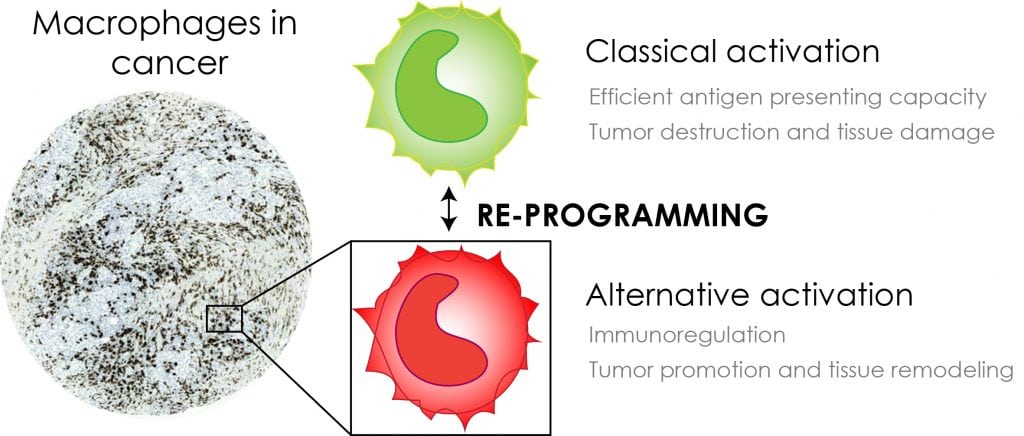
This controversy arises from the functional plasticity of monocytic phagocytes, which express pro- or anti-tumoral functions in response to different microenvironmental signals (Fig. 1). During initial carcinogenesis, the tumour-infiltrating myelomonocytic cells represent the classically activated macrophages with cytotoxic activity towards cancer cells. They have high antigen presenting capacity and high interleukin (IL-12 and IL-23) production, and they release high levels of reactive oxygen intermediates and tumour necrosis factor-alpha (TNFα). As tumours develop TAMs become alternatively activated in response to high levels of colony-stimulating factor-1 (CSF-1) and immunosuppressive cytokines such as IL-4 and IL-10 expressed by malignantly transformed cells or other stromal compartments.
Macrophages as targets for cancer immunotherapy
Tumour-associated macrophages are highly eligible candidates for targeted cancer immunotherapy, since these cells are abundantly present in various tumours, they are very plastic and can be converted into pro-inflammatory macrophages supporting T-cell activation and tumour rejection.
Current approaches targeting macrophages include:
- Depletion of macrophages;
- Stimulation of macrophages;
- Inhibition of macrophage trafficking into tissues; and
- Reprogramming of macrophages.
To date, the majority of macrophage-targeted strategies under clinical development utilise macrophage colony-stimulating factor receptor inhibition to deplete or reprogramme macrophage populations in tumours. Despite promising results in several preclinical studies, the clinical efficacy of CSF-1R blockage as monotherapy has been rather disappointing.
It is important to note that macrophage depletion strategies have a context-dependent shortcoming since they can also promote depletion of pro-inflammatory macrophages, which mediate favourable anti-tumour immune responses. In fact, we have shown that anti-CSF-1R therapy promotes depletion of lymph node subcapsular sinus macrophages in a triple-negative breast cancer model.2 The subcapsular sinus-lining macrophages are the gatekeepers for metastasising cancer cells and promote immune recognition of foreign antigens via T-cell activation.
In addition to this, several studies have reported resistance to CSF-1R inhibition in various tumour models because of the presence of other factors affecting macrophage survival and differentiation. Thus, there is an urgent need to find better ways to utilise these cells as an armour to fight against cancer.
Reprogramming of macrophages – a clever approach to cancer immunotherapy
Both activating and suppressing immune responses are needed in maintaining proper homeostasis of the body. Interestingly, during pregnancy an immunosuppressive milieu prevails in the placenta to tolerate the growing foetus. Similarly, the developing tumour harnesses the immune system for its own development. A common feature of both situations is the high abundance of Clever-1 positive macrophages, which support the formation of an immunosuppressive tissue microenvironment.
What is Clever-1?
Clever-1, also known as Stabilin-1 and Feel-1, is a multifunctional molecule conferring scavenging ability on a subset of alternatively activated macrophages. In these cells, it is involved in receptor-mediated endocytosis and recycling, intracellular sorting, and transcytosis of altered and normal self-components. Although other leukocyte types (granulocytes and lymphocytes) are Clever-1–negative, it is also constitutively expressed on:
- Afferent and efferent lymphatic endothelial cells;
- Sinusoidal endothelial cells in the liver and spleen; and
- High endothelial venules.
Moreover, upon inflammation, it can be induced on blood vessel endothelium, where it mediates the trafficking of lymphocytes, granulocytes, and monocytes from the blood into the inflamed tissue.
The scavenging functions of Clever-1 are relatively well defined, but its association with immune regulation is poorly known. The feasibility of targeting Clever-1 to alleviate tumour-related immunosuppression is strongly supported by different experimental tumour models and current knowledge regarding the association of its expression to the poor clinical outcome of cancer.3,4 The development of Clever-1 as a companion diagnostic and prognostic biomarker is highly useful for personalised therapy, and the results of our research are expected to promote anti-Clever-1 therapy into clinical use for those suffering from refractory, untreatable cancers.
The current working hypothesis from our lab suggests that Clever-1 is an immunoswitching molecule on anti-inflammatory macrophages, and by modulating its functions by antibody targeting it is possible to reprogramme macrophages as an additional strategy to promote anti-tumour immunity. This is highly important since efforts to reactivate immune responses against tumours solely by blocking T-cell inhibitory molecules have not shown promise as a single therapy for the vast majority of patients.
Translating cancer immunotherapy from bench to bedside
Significant discoveries in the field of tumour immunology have revolutionised cancer treatment in the last few years and are now offering unforeseen prospects of cure for some patients. Despite these advances, up to 80% of patients remain refractory to current immune checkpoint inhibitor therapy for mainly unknown reasons. Tumours can be immunologically classified (inflamed (hot) – non-inflamed (cold)) based on the presence of tumour-infiltrating cytotoxic CD8 T-cells. The inflamed tumours show high mutational load, high interferon γ (IFNγ), and programmed death ligand 1 (PD-L1) expression and respond favorably to immune checkpoint blocking therapies.
By modulating Clever-1 functions we hope to help the majority of patients lacking response to immune checkpoint blockage by converting cold tumours to hot ones. We work together with Faron Pharmaceuticals Ltd to support the development of their lead compound CLEVEGEN into clinical trials. CLEVEGEN is a humanised IgG4 that targets a functional epitope on Clever-1.
What is MATINS?
The MATINS (Macrophage Antibody to FP-1305 To INhibit immune Suppression) study is a
first-in-human open label Phase I/II adaptive clinical trial (sponsored by Faron Pharmaceuticals) in selected metastatic or inoperable solid tumours to investigate the safety and efficacy of CLEVEGEN (FP-1305), the humanised monoclonal antibody against Clever-1. The selected tumours are cutaneous melanoma, hepatobiliary, pancreatic, ovarian or colorectal cancer, which are all known to contain high amounts of Clever-1-positive TAMs.
The trial is to be run in three parts. Part I will be conducted to determine the safe and tolerable dose of CLEVEGEN, which will then be used in Part II to expand the cohorts of individual tumour types. Part III of the trial aims to confirm the efficacy of CLEVEGEN with the cohorts selected based on Part II.
The possibility of probing the molecular landscape of solid tumours via a blood draw (liquid biopsy) has attracted remarkable interest among the oncology community. Our group aims at identifying patients under immunosuppression who could benefit from immunoswitching therapies. To date, we have detected increased expression of Clever-1 on peripheral monocytes (the precursors of TAMs) in cancer patients. This provides the benefit of patient selection during the MATINS trial as an indicator of prevailing immunosuppressive inflammation. We find this highly important since patient selection is central to the success of targeted therapies and identification of tumour-specific molecular landscapes is pivotal to guiding treatment choices. Longitudinal surveillance of anti-tumour immune responses is essential for precision medicine but cannot be effectively achieved using tissue biopsy specimens.
The MATINS trial offers our group a unique possibility to investigate the inflammatory responses related to the release of immunosuppression in tumours before, during and after CLEVEGEN therapy and identify markers of response for future drug development. Future cancer treatments will involve combination therapies, and we think that CLEVEGEN could bring a synergistic benefit, for example, to anti-PD-1/PD-L1 and increase the success rate of cancer immunotherapy.
References
- Characterization of macrophage – cancer cell crosstalk in estrogen receptor positive and triple-negative breast cancer. Hollmén M, Roudnicky F, Karaman S, Detmar M. Scientific Reports 3/2015 doi:10.1038/srep09188
- G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Hollmén M, Karaman S, Schwager S, Lisibach A, Christiansen AJ, Maksimow M, Varga Z, Jalkanen S, Detmar M. Oncoimmunology 11/2015, doi: 10.1080/2162402X.2015.1115177
- Clever-1/Stabilin-1 Controls Cancer Growth and Metastasis. Karikoski M, Marttila-Ichihara F, Elima K, Rantakari P, Hollmén M, Kelkka T, Gerke H, Huovinen V, Irjala H, Holmdahl R, Salmi M, Jalkanen S. Clin Can Res 10/2014 doi: 10.1158/1078-0432.CCR-14-1236
- Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundström J, Salmi M, Ristamäki R, Jalkanen S. Int J Cancer 8/2012, doi: 10.1002/ijc.26457
Maija Hollmén, PhD
Group Leader, Academy Research Fellow, Adjunct Professor of
Tumor Immunology
MediCity Research Laboratory, Institute of Biomedicine, University of Turku
+358 50 514 2893
maija.hollmen@utu.fi
Tweet @maijahollmen/
@HollmenMaija
hollmenlab.utu.fi
This is a commercial article that will appear in Health Europa Quarterly issue 6, which will be published in August, 2018.









