
A research team from Hokkaido University has utilised gene editing technology to identify new molecular targets for treating aggressive forms of leukaemia in adults.
What is leukaemia?
Adult T-cell leukaemia/lymphoma (ATLL) is an aggressive form of blood cancer that is linked to a viral infection. Treatment for ATLL is different forms of chemotherapy, which have been found to be particularly ineffective in treating this disease— the long-term survival rate is only 20%. A significant amount of research has been conducted to explore the molecular pathways and gene mutations involved in this cancer, but these vary between patients, thus making it difficult to develop effective treatments.
How was this gene editing technology developed?
Masao Nakagawa, haematologist at Hokkaido University, and colleagues in Japan, the US, and France utilised the CRISPR-Cas9 gene editing technology to inactivate thousands of genes in several ATLL cell lines. Their intention was to pinpoint the genes and pathways that could be targeted with drugs to kill ATLL cells.
This process led to the identification of 1,278 genes that were essential for the survival and proliferation of ATLL cells. Then, they narrowed down this number to nine genes for more detailed analysis.
This research study was recently published in the journal Blood and was funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP18K08313, JP21H02775); the SENSHIN Medical Research Foundation; the Takeda Science Foundation; the EU Transcan-2 consortium ERANET-PLL; the National Institutes of Health, National Cancer Institute (CA100730), and the Centre for Cancer Research.
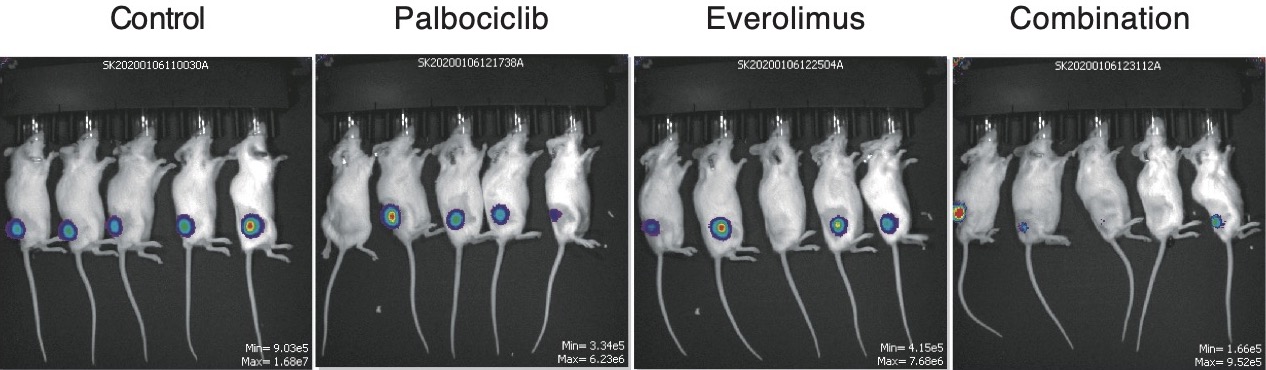
What did the findings from this experiment reveal?
Among the 1,278 genes that were studied, scientists discovered that one gene in particular proved promising as a drug target. The gene CDK6 encodes an enzyme that activates cell proliferation. The researchers found that inactivating the enzyme with a drug currently utilised to treat breast cancer – known as palbociclib – cell division stopped and caused cell death in some, but not all, of the tested ATLL cell lines.
The cell lines that were resistant to the drug had a mutation in a gene called TP53, which under ordinary circumstances is involved in preventing tumour growth and development. These cells died when treated with palbociclib that had been combined with an activator of mutant TP53 – a drug that is currently utilised to treat a type of blood cancer called myelodysplastic syndrome.
Additionally, it was discovered that combining palbociclib with the drug everolimus – which inhibits a protein complex (mTORC1), is involved in protein synthesis for cell growth and proliferation and is used to treat kidney and breast cancer – also killed ATLL cells, including those with TP53 mutations. The drug combination also considerably reduced tumour growth and had minimal side effects when tested in mice with grafted ATLL tumours.
“Our study reveals CDK6 as an attractive target for treating ATLL,” concluded Nakagawa. “However, we also discovered that loss of TP53 function in some ATLL cell lines leads to resistance to the CDK6 inhibitor, palbociclib. Given this finding, researchers should consider the status of TP53 in future clinical trials of palbociclib in ATLL.”









