George Stantchev, PhD, the CEO of COMERG, provides a detailed and insightful analysis of the safety of utilising R134a in cannabis extraction.
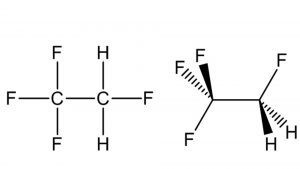
Hydrofluorocarbon HFC-134a (R-134a), also known as 1,1,1,2-tetrafluoroethane, is a gaseous halocarbon that is considered a prime candidate for replacing other chlorinated halocarbon materials, such as Freon 12 and Freon 22, for use in air conditioning and refrigeration systems and as an aerosol propellant or foam expansion agent. This change is due to a federal regulation that mandates switching from CFCs to other suitable compounds that do not damage the ozone layer.
The real question is: if HFC-134a is utilised to extract cannabis and there is residual gas in the form of a liquid in the extract, what are the potential safety concerns? For human consumption in the form of ingestion, there is an FDA exemption for flavours and flavourings for foods that state that a maximum of 1000ppm of residual can be present for human consumption. Albeit alternate industry universe, the same should apply to cannabis edibles. As a function of design, our extraction platform has been characterised with the maximum residual solvent of approximately 800ppm, therefore below the established threshold value of 1000ppm.
GRAS EXEMPTION CLAIM
INEOS Fluor Ltd. hereby claims that the use of 1,1,1,2-tetrafluoroethane (HFC-134a) as an extraction solvent in the production of flavours and flavourings for foods is exempt from the premarket approval requirements of the Federal Food, Drug and Cosmetic Act because we have determined that such use of HFC-134a is generally recognised as safe (GRAS). The maximum specification residue in the food flavour extract is 1000 ppm
For inhalation via smoking, the above does not apply. All extracts for smoking processed utilising HFC-134a will go through a degassing process as part of the SOP, and within 24 hours, residual solvent is potentially removed to a non-detect state. Below you will read that most vape pens, dab rigs or nails will operate in the range of 230 to 300°C (450 to 570°F) to vaporise the smoke for inhalation. Remember, the combustion of cannabis (THC) occurs at 230°C (450°F).
Industry Uses for R134a
Refrigeration
1,1,1,2-Tetrafluoroethane is a non-flammable gas used primarily as a “high-temperature” refrigerant for domestic refrigeration and automobile air conditioners. These devices began using 1,1,1,2-tetrafluoroethane in the early 1990s as a replacement for the more environmentally harmful R-12. Retrofit kits are available to convert units that were originally R-12-equipped.
Aerosol
For its medical uses, 1,1,1,2-tetrafluoroethane has the generic name norflurane. It is used as a propellant for some metered dose inhalers. It is considered safe for this use. In combination with pentafluoropropane, it is used as a topical vapocoolant spray for numbing boils before curettage. It has also been studied as a potential inhalational anaesthetic, but it is non-anaesthetic at doses used in inhalers.
Propellant
1,1,1,2-Tetrafluoroethane has also been used to cool computers in some overclocking attempts, such as crypto coin mining. It is the refrigerant used in plumbing pipe freeze kits. It is also commonly used as a propellant for airsoft air guns in substitution for CO2. The gas is often mixed with a silicone-based lubricant and is also used as a propellant in building insulation foams along with R-245.
Solvent
1,1,1,2-Tetrafluoroethane is also being considered as an organic solvent suitable for extraction of flavour and fragrance compounds, as a possible alternative to other organic solvents and, more specifically, the supercritical carbon dioxide. It can also be used as a solvent in organic chemistry, both in liquid and supercritical fluid form.
Cannabis processing
R134a is much less miscible with the extracts than butane is. The standard protocol for post-processing with butane is several days in a vacuum oven to purge the dissolved butane. There is a half-hour to an hour purge of the R134a built into the vendor’s process, which reduces the concentration to very low levels.
It has been a rumour in the industry that in very high temperatures, R-134a decomposes to hydrogen (HF) and carbonyl fluoride (CF), which may cause irritation when inhaled. We are going to be reviewing this topic based on scientific data on the actual effects. In general, direct contact of 1,1,1,2-tetrafluoroethane with open flames or hot surfaces in excess of 370°C (698°F) may cause vapour decomposition and the emission of toxic gasses, including hydrogen fluoride and carbonyl fluoride.
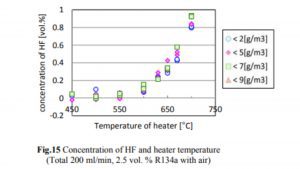
A 2014 study done at Perdue found essentially no decomposition of R134a to HF until the temperature exceeded 550°C, which is much hotter than the temperatures at which hash oil is typically consumed. The concentrations of R134a, HF, and CF in the case of R134a testing are shown in Figs. 14, 15, and 16.
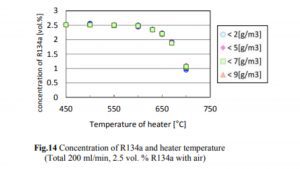
The decrease in the R134a concentration and the increase in the HF concentration began when the heater temperature was in the range of 500–550°C, and the generation of CF began when the temperature exceeded 650°C. The decrease in the R134a concentration was not affected by the humidity.
As one can see, the conversion of R134a to HF starts at 600°C with a rate of 0.01% or 100ppm. Keeping in mind that a butane flame temperature is 300°C while dabbing, there will be no conversion of R134a to HF occurring at that temperature. Although if we assume that 600°C is achieved (which is 300°C over the actual dabbing temperature) somehow and if the allowed concentration for R134a in an extract is below 20ppm, we are talking about max HF conversion of 0.2ppm (400ppb).
According to NIH data, the irritation from HF occurs at levels higher than 3ppm with exposure for over one hour. The max anticipated conversion will be 15 times below the limit, besides the fact that the dabbing temperature of a pin is 300°C below the conversion temperature. The critical effects of inhalation exposure to hydrogen fluoride are respiratory tract irritation and the induction of respiratory disease. Respiratory tract irritation is documented in animal models and has been observed in controlled human exposure studies.
According to the above, the sensory irritation can occur at exposures greater than 3ppm for one hour (Lund et al. 1997). Prolonged respiratory tract effects can occur after short-term exposure. To evaluate longer-term exposures or systemic effects, the total fluoride intake from all exposure routes (inhalation, dermal, and ingestion) must be considered (EPA 1988; NRC 2006). Chronic exposure is a repeated exposure lasting more than approximately 10% of the lifespan in humans or approximately 90 days to two years.
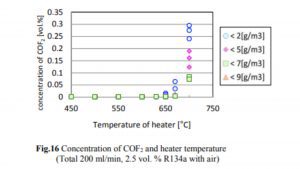
As one can see, the conversion of R134a to CF starts at 650°C with a rate of 0.02% or 200ppm. Keeping in mind that a butane flame temperature is 300 C while dabbing, there will be no conversion of R134a to HF occurring at that temperature. Although if we assume that 650°C is achieved (which is 350°C over the actual dabbing temperature) in some way and if the allowed concentration for R134a in an extract is below 20ppm, we are talking about a max HF conversion of 0.4ppm (400ppb).
NIH data states that the irritation from CF occurs within the recommended exposure limit is 10 Hour Time-Weighted Avg 2 ppm (5 mg/cu m). The max anticipated conversion will be five times below the limit, besides the fact that the dabbing temperature of a pin is 300°C below the conversion temperature.
Fire extinguisher agent
1,1,1,2-Tetrafluoroethane is also being considered as an alternative to sulfur hexafluoride as a dielectric gas. Its arc-quenching properties are poor, but its dielectric properties are fairly good. It has been used as an alternative to sulfur hexafluoride in magnesium smelting as a shielding gas.
Other uses
Other common uses include a propellant for the delivery of pharmaceuticals (e.g. bronchodilators), wine cork removers, gas dusters (“canned air”), and air dryers for removing the moisture from compressed air, plastic foam blowing, as a cleaning solvent, etc. It is used in the resistive plate chamber particle detectors in the Large Hadron Collider. It is also used for other types of particle detectors, such as some cryogenic particle detectors.
Dosing
The US Navy requested that the NRC review the toxicity data on HFC-134a and recommend one-hour and 24-hour EEGLs. The Navy also requested that the NRC recommend a 90-day CEGL for HFC-134a and identify appropriate research to fill data gaps. We have collected the available supporting documentation and the above evaluation in support of the dosing.
In general, no toxicity data are available on humans following exposure to HFC-134a. Animal studies indicate that HFC-134a has a low level of systemic toxicity following acute, subacute, subchronic, and chronic exposures. For example, neurotoxicity and cardiac sensitisation occur after acute exposures to HFC-134a at very high concentrations.
R134a Bans
In 2012, automobile manufacturers began the transition to new, climate-friendly alternative refrigerants. As a result of a July 2015 rulemaking, by the model year 2024, the MVAC systems in newly manufactured light-duty vehicles in the United States will no longer use HFC-134a. The HFO refrigerant R1234yf, designed as a replacement for R134a in car air conditioning systems, is extended for use in newly manufactured medium-duty passenger vehicles, heavy-duty pickup trucks, and complete HD vans.
There was an indent of US EPA to ban several common refrigerants, including R134a, R410A and R407C, which will be banned from use in new chillers in the USA from January 1, 2024. The wide-ranging changes also place future restrictions on using the higher GWP HFC gasses in new domestic and commercial refrigeration equipment and ban the use of class 3 flammable refrigerants as retrofits. All those bans are applied in the light vehicle consumer refrigeration applications and will not affect the commercial use of the R134a.
References
- https://en.wikipedia.org/wiki/1,1,1,2-Tetrafluoroethane– R134A
- https://www.ncbi.nlm.nih.gov/books/NBK231519/– R134A
- https://www.nj.gov/health/eoh/rtkweb/documents/fs/0348.pdf– Carbonyl Flouride
- https://www.ncbi.nlm.nih.gov/books/NBK219903/– Hydrogen Fluoride
- https://en.wikipedia.org/wiki/Median_lethal_dose– Toxicity Scale
- https://en.wikipedia.org/wiki/1,1,1,2-Tetrafluoroethane– Tetrafluoroethane
- https://en.wikipedia.org/wiki/Carbonyl_fluoride– CF.
- https://en.wikipedia.org/wiki/Hydrogen_fluoride– HF
- https://en.wikipedia.org/wiki/1,1,1,2-Tetrafluoroethane_(data_page)– data page
- https://en.wikipedia.org/wiki/2,3,3,3-Tetrafluoropropene– R1234yf
- https://www.coolingpost.com/world-news/us-confirms-r134a-chiller-ban-in-2024/– bans
- https://www.epa.gov/mvac/refrigerant-transition-environmental-impacts– vehicles
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/1-1-1-2-tetrafluoroethane