Angany Inc is an emerging Franco-Canadian pharmaceutical company which aims to bring new hope to people suffering from allergies.
The word ‘angani’ means ‘open horizons’ in Swahili. The scientists at Angany have developed a new approach to allergy immunotherapy and were just about to start testing their first allergy vaccine candidate in human trials when the pandemic struck.
The situation became compelling and called for immediate action. In a quick turnaround, Angany was able to leverage the potential of its innovative immunotherapy and biomanufacturing platforms in order to design and produce a preventive COVID-19 vaccine and a broad-spectrum anti-SARS-02 monoclonal antibody cocktail.
The current paradigm of allergy treatment
Allergies affect around a third of the global population, who suffer from a variety of symptoms, health issues, diseases or life-threatening conditions directly related to allergic reactions. Allergy is now on the World Health Organization’s (WHO) list of the most significant global health threats, and its incidence has been increasing for the last 20 years.
If you suffer from allergies, there is a very strong probability that you did not choose allergy immunotherapy (AIT) as a preferred treatment option; fewer than 5% of allergic people do. Chances are that you deal with your condition either by avoiding contact with the allergen source – sources may include pollen, cats or specific foods such as peanuts – as much as possible, or through the use of over-the-counter (OTC) or prescription antihistamines and other symptomatic drugs.
The other option available to patients, allergen immunotherapy, involves embarking on a long and invasive desensitisation protocol, which comes with a prognosis of slow and somewhat unpredictable buildup of tolerance to the source of the allergy. When patients do enter such a protocol, there is also a high probability that they will not continue treatment for the full planned duration – fewer than 30% of patients complete allergen immunotherapy treatment in full – and that their symptoms will eventually return. In addition, the treatment is not available to all allergy patients: for instance, allergen immunotherapy was not on the list of treatment options for patients who are allergic to food items, such as peanuts, until the recent launch of oral delivery desensitisation products.
Symptomatic drugs for food allergies, such as epinephrine auto-injectors, function as heavy artillery, as food allergens provoke systemic reactions and can cause anaphylaxis. It is generally accepted that allergies could be better treated if accurate diagnosis and adapted treatments were made possible in infancy. The field of allergy medicine still lacks both a precise, reliable diagnosis of allergy and corresponding efficacious immunotherapeutic treatment in an appealing administration regimen.
Angany’s new approach to allergy treatment
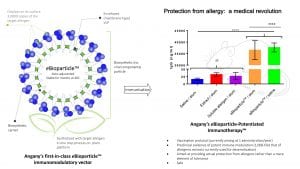
Angany Inc is a young Franco-Canadian pharmaceutical company headquartered in Québec City, Canada. The company operates a pilot production unit in Lévis, Québec, Canada and a research and development laboratory in Val-de-Reuil, Normandy, France. The company has been developing and testing a totally new approach to the treatment of allergies. Its approach is based on a new generation of recombinant allergen nanoparticles which are in vivo synthesised in, and purified from, plants. Plants are not the only system capable of producing bioparticles, but Angany has been using this specific system for its key benefits in terms of safety, yield, ease of scale-up and implementation, and low capital expenditure.
The allergen bioparticles developed and produced by Angany will be used in allergy vaccines, rather than in desensitisation programmes. Although the specific details of the administration regimen have yet to be confirmed through clinical trials, the results so far, both in sensitised animal models and ex vivo in sera from allergic patients, show that the particles are strongly immunogenic; that they trigger a Th1/Treg immune response with a strong innate component; and that they remain hypoallergenic at doses 1,000 times higher than natural allergens. Angany’s allergen bioparticles are stable, biosynthetic proteolipid spheres of approximately 150nm in diameter, with more than 3,000 copies of a single allergen present on their surface. These bioparticles mimic viruses in their interaction with the immune system, and thus trigger a rapid and massive humoral and cellular response which has the power to completely neutralise the targeted allergen upon subsequent exposure.
A young company with gusto and experience
Angany Inc is a young company, but it is not without experience. Its current CEO, Dr Louis-Philippe Vézina, is known worldwide as an expert in the development of new vaccine platforms; and has already seen success in bringing a new generation of seasonal vaccines to clinical development and full-scale production as Chief Scientific Officer of Canadian biopharmaceutical developer Medicago inc. Dr Vézina is assisted by a seasoned team of executives with experience and demonstrated expertise in vaccinology, protein chemistry, protein maturation and glycosylation, industrial process development, current good manufacturing practice (cGMP) manufacturing, clinical development and regulatory affairs. Angany is also supported by several key high-profile opinion leaders and allergy clinicians from the world over.
Angany’s first entry into clinical development, set to launch in 2021, will be a candidate vaccine for human allergy to cats, soon to be followed by a candidate vaccine for peanut allergy (Q1 2022). As these will be entered into clinical protocols as vaccines and not as desensitisation products, and as clinical trials in allergy are typically performed on allergic patients, it is expected that Angany will be able to provide safety and preliminary efficacy results as early as 2022 for its two first vaccine candidates.
Angany’s response to the COVID-19 threat
Angany’s agressive development plan was significantly challenged by the emergence of the SARS-Cov-2 pandemic. “We were just about to enter pre-clinical trials with our first allergy vaccine when the pandemic struck,” says Dr Vézina. “Our first reaction was obviously one of profound disappointment, but we also realised that we had two extraordinary technologies to bring upfront in the fight against the SARS virus. With our bioparticle platform, we had the unique ability to design and develop a receptor-binding domain (RBD)-specific prophylactic vaccine; something that no other known vaccine platform could do. With our plant-based manufacturing platform, we had the ability to produce SARS-CoV-02-neutralising monoclonal antibodies (NAbs) with a surge capacity none of the other platforms could match.”
A little more than 12 months later, Angany is still involved in the COVID-19 effort. Its prophylactic vaccine did not make it to the finish line as a result of lack of visibility in the vaccine industry at that time, and subsequent lack of financial support. The relevance of Angany’s novel approach to vaccine development is still undisputed; and could still be harnessed in the production of new vaccines for the coming waves of variants. The relevance of Angany’s plant-based manufacturing platform is increasingly being noticed and valued. The world is looking for multivalent NAb cocktails that can provide protection against emerging variants, as well as for new production capacity for these NAbs – and Angany can provide both.
As early as June 2020, Angany had entered into business discussions with a high-profile international consortium which had isolated NAbs with remarkably high potency against the SARS-CoV-02 wild-type strain. These discussions concluded with the formation of a partnership for the development of a bivalent NAb cocktail produced in plants; and the results from this partnership became tangible as soon as December 2020. Angany and its partner are now working on a third valence for this cocktail, which they plan to move into clinical trials in the coming months. This cocktail has a broad and strong affinity for the primary COVID-19 strain and two of its variants.
As this NAb cocktail can be produced in plants at a fraction of the cost that would be expected from more traditional manufacturing approaches, and as implementation of a full-scale upstream plant-based manufacturing facility is a matter of months, compared to years for bioreactors, Angany’s proposal has begun to attract attention from many countries which now feel the urge to secure their own autonomy in the manufacturing of the biopharmaceuticals essential to fighting a pandemic: vaccines and monoclonal antibodies.
Now is Angany’s time to shine
The challenge of the pandemic has strengthened Angany’s team of scientists and biopharma professionals. The development milestones met over recent months have only added to the confidence and determination of a passionate team. The company hopes its work will soon bring health and a better life to people suffering from allergies. There is no doubt a renewed sense of mission for the team, brough on by seeing how the technological platforms it has developed can play a crucial role in so many other domains of health.
This article is from issue 17 of Health Europa. Click here to get your free subscription today.













